NMR Practice Problems
In the following examples, we will learn how to solve NMR practice problems step-by-step in over 100 min video solutions which is essential for organic structure determination.
The emphasis is on the 1H proton NMR and most problems are based on understanding its key principles such as the number of NMR signals, integration, signal splitting (multiplicity), and, of course, the strategies of putting all of these together to come up with the correct structure.
Aside from the 1H NMR, we will also go over determining the hydrogen deficiency index (HDI), solving problems where the 13C NMR, DEPT, and IR are given along with the 1H NMR spectrum.
The problems are chosen to demonstrate the most common patterns in 1H NMR spectroscopy, as well as, the situations where you need to consider the possibility of signal overlapping, incorrect absolute values of integrations, as the instrument measures only the relative area for each peak, examples where fairly large molecules give rise to spectra with few signals because of the symmetry elements. We will also discuss the purpose of shaking the sample with deuterated solvents.
How to determine the structure of an unknown compound using NMR spectroscopy
Two things you will need to do very often:
- HDI – In general, most problems will give you the molecular formula of the unknown and you must know how to determine the hydrogen deficiency index. Here is the formula for this:
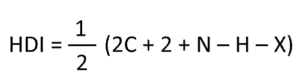
but you can also learn how to do this without the formula (read about hydrogen deficiency index here). - IR – It will be very beneficial for you to remember key signals in IR spectroscopy so that you can identify the functional groups. Solving IR problems – here.
Before you start working on the first problem or getting stuck on one, let’s go ahead and summarize the most common patterns that you need to recognize.
Common Splitting Patterns in NMR spectroscopy
Start by analyzing the aliphatic region. Many molecules have at least one methyl group and depending on its environment, common splitting patterns are observed.
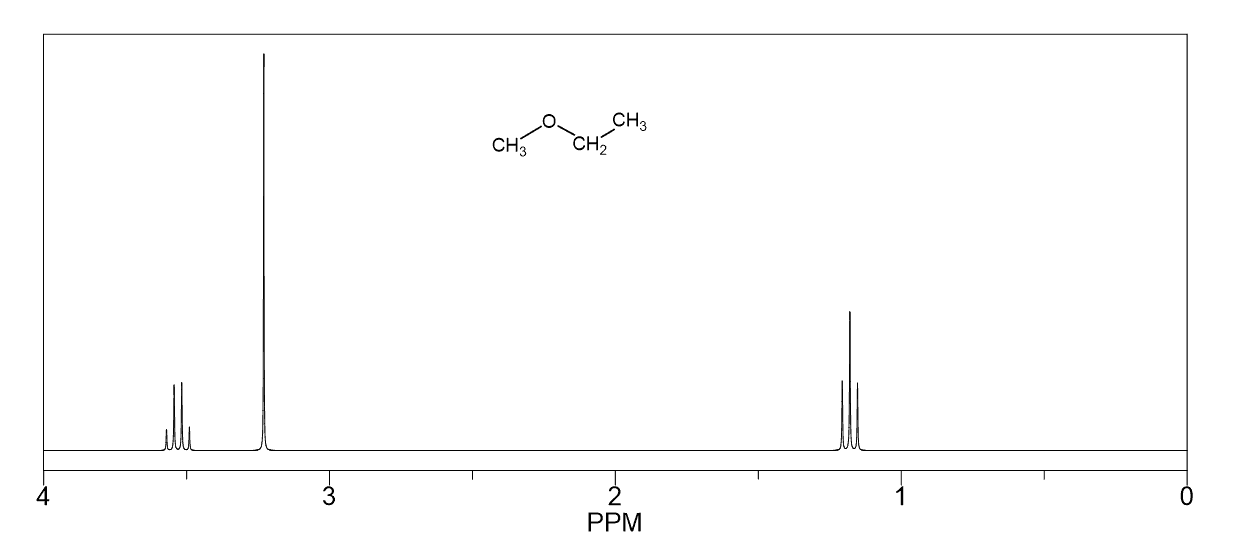
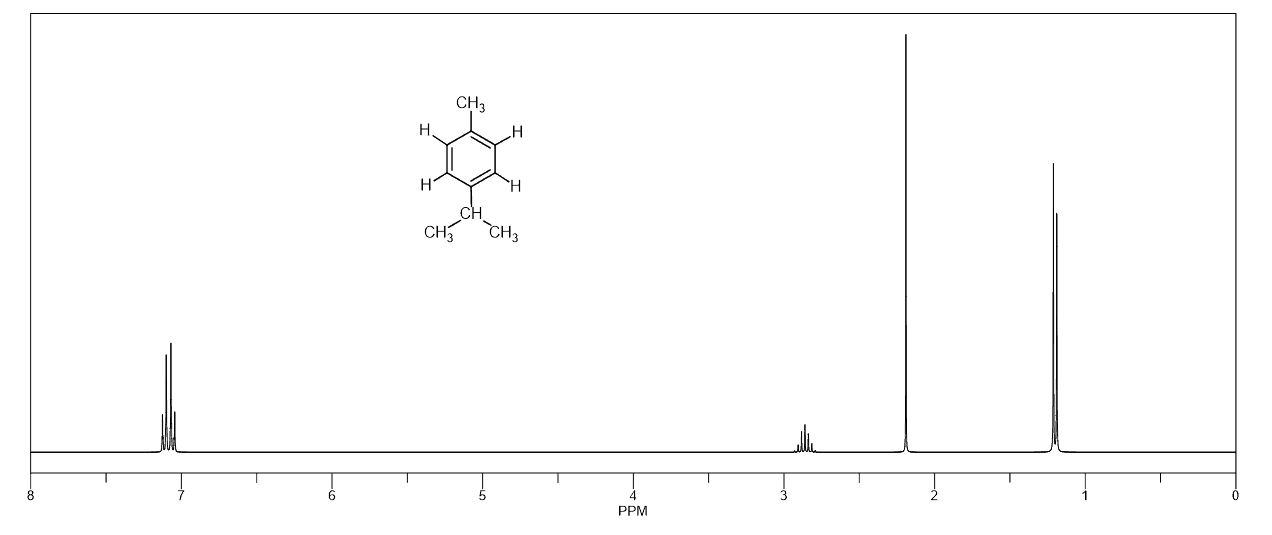
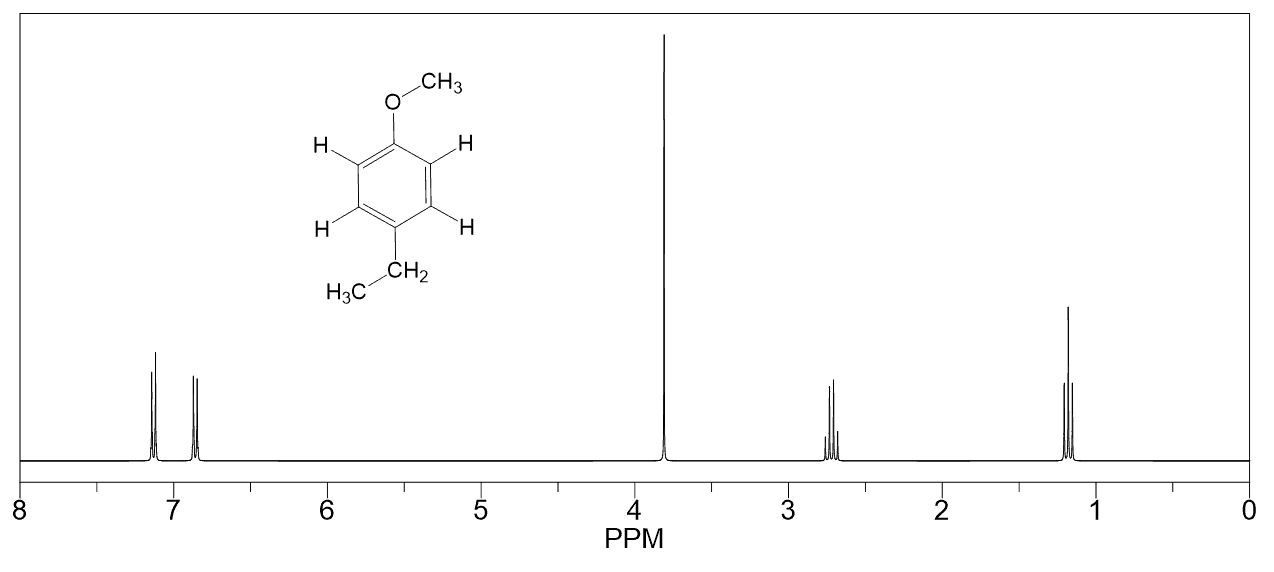
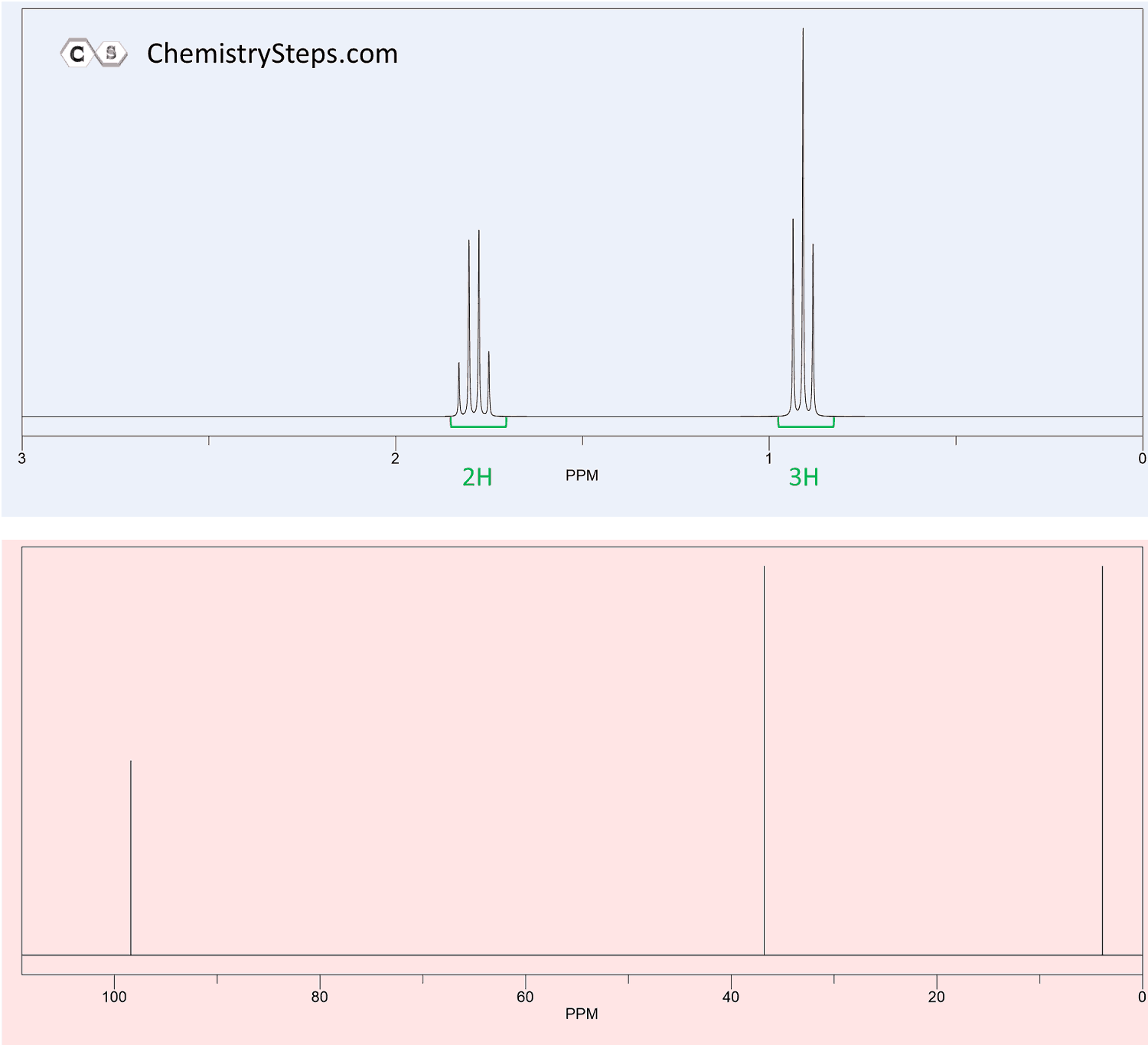
A triplet with an integration of three represents a methyl with an adjacent CH2 group. So, look for a triplet and a quartet with integrations 3 and 2 which indicates an ethyl group (Figure 1a).

If there is a singlet with an integration of 3, this represents an isolated methyl group (b). On the other hand, if the singlet integrates to 9 protons, this indicates a tert-Butyl group (c)
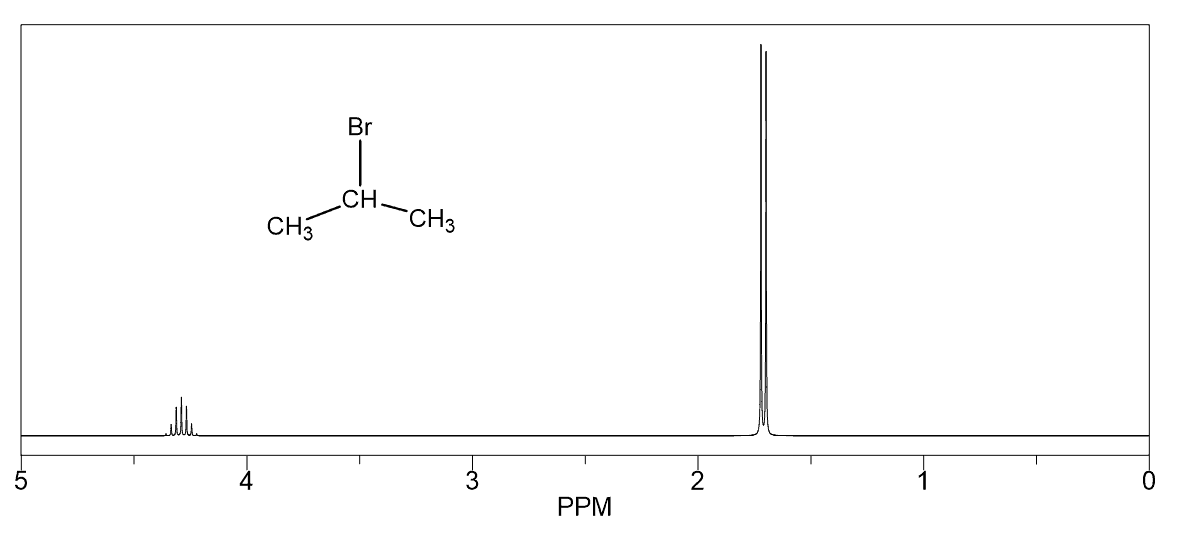
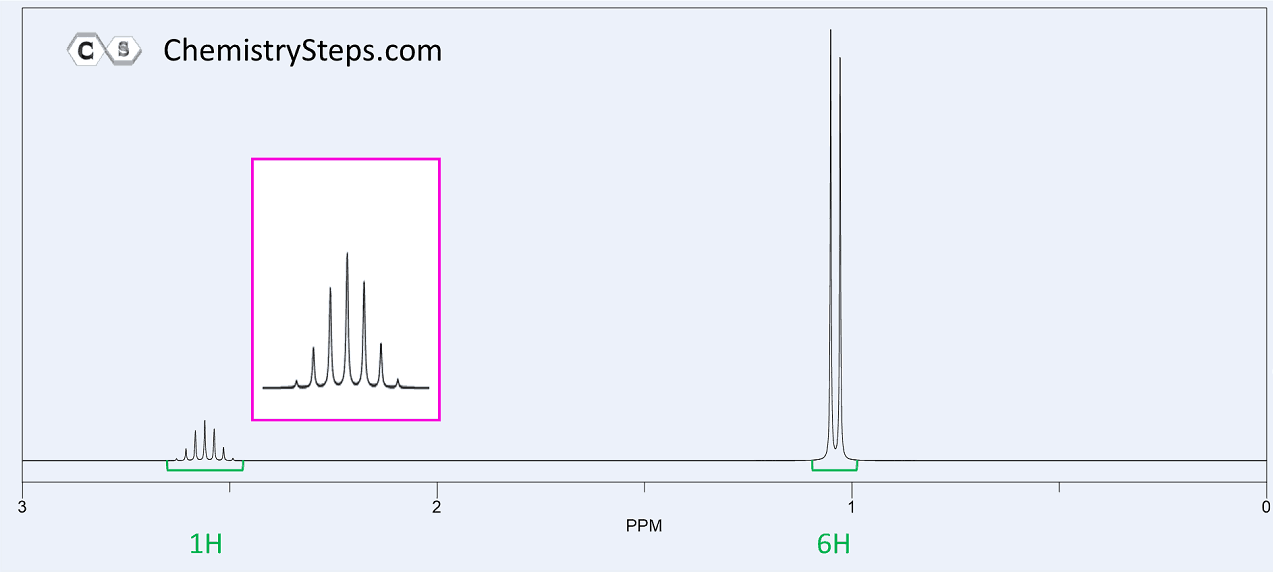
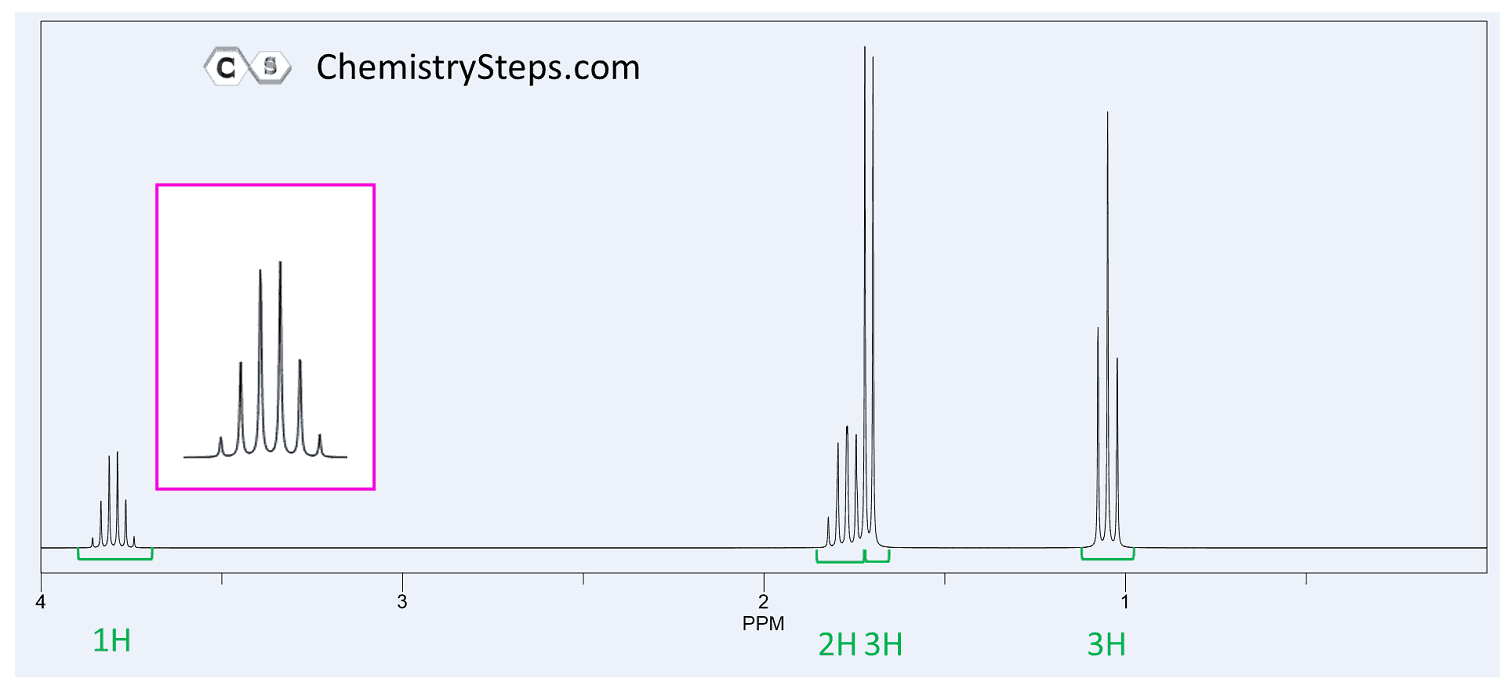
Another common fragment is the combination of a doublet (6H) and a septet (d). This indicates an isopropyl group where the methyl protons are split into a doublet by one adjacent proton and the six equivalent protons of the methyl groups split the signal of that one proton into a septet.
If you see a bunch of triplets each integrating to two protons, think about the -CH2-CH2– ethylene group (e).
Other Regions and Splitting Patterns
Alkenes
Alkene protons appear at ~5-6 ppm and you can recognize them by the small integration (1 or 2) and complex splitting. This is because all the protons on a double bond are capable of splitting each other and the coupling constants vary anywhere from 5-18 Hz.

Aromatic
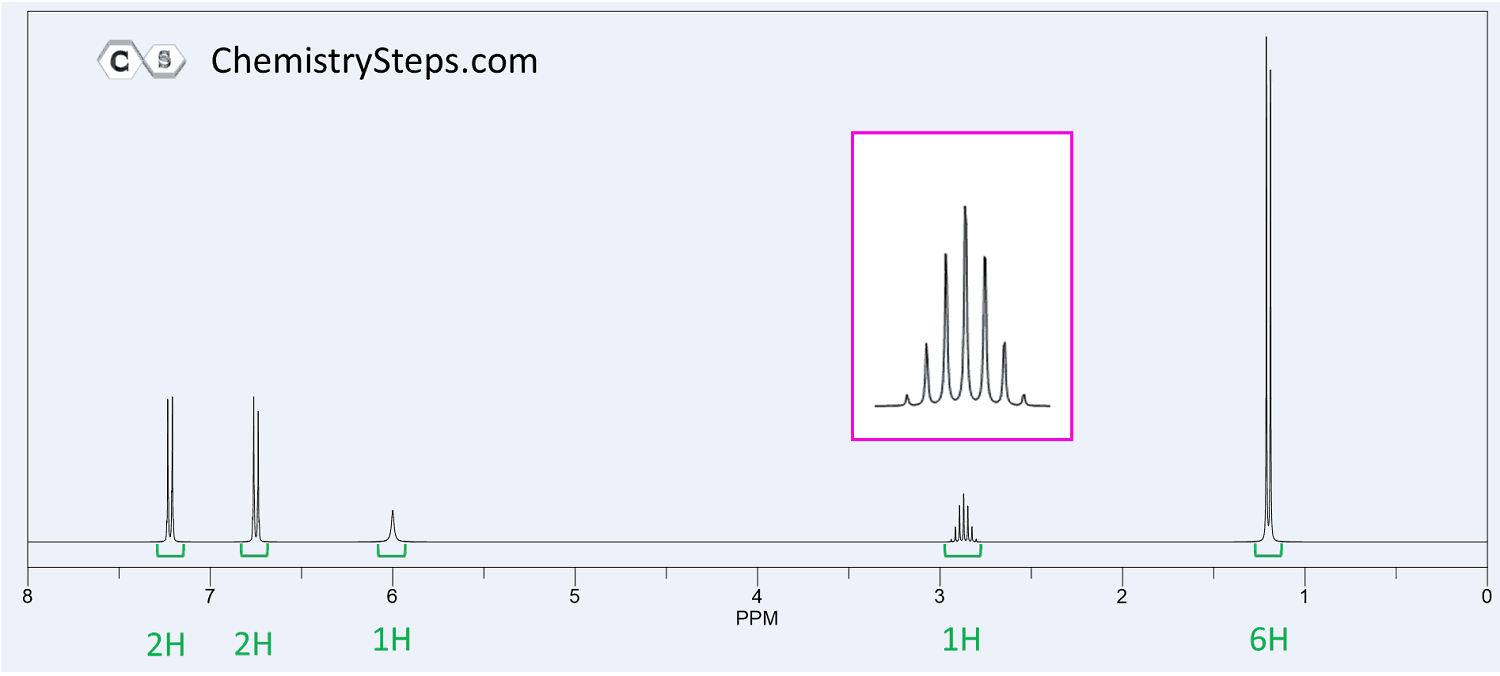
Aromatic protons appear at the ~7-8 ppm region. If you have a signal there, the first thing is to check how many groups (or how many protons) are on the aromatic ring. And this is done simply by looking at the integration:

One common splitting pattern in the aromatic region is the presence of two doublets each integrating to two protons. This indicates a symmetrically substituted ring with two groups (1, 4- or para– substituted).

Most other substitution patterns give complex splitting and the easiest for you will be looking at the total integration of all the aromatic signals.
Aldehydes
Next functional group recognizable in 1H NMR spectroscopy is the ~10 ppm signal of aldehydes. Usually, it shows up as a singlet, though splitting with adjacent protons is not uncommon either:

Another way of identifying aldehydes and ketones as well as checking for a ~200 ppm signal on the 13C NMR. Esters and carboxylic acids appear less downfield – 160-180 ppm.
The OH peak
Like for the IR spectroscopy, the OH peak is a good indicator here as well. Look for a broad peak anywhere from 1-6 ppm. Most often though it will be in the 4-6 range.

Remember also that the OH signal is not split by adjacent protons unless the sample is very dry.
Other groups that give broad, and sometimes, deuterium-exchangeable signals are the amines, amides, and thiols.
Carboxylic acids
If you see a broad signal at 12 ppm, 90% of the time it tells you about a carboxylic acid.

In the following NMR practice problems, we will go over the best strategies you can use for identifying the structure of unknown compounds. As a Chemistry Steps Prime member, you will also get access to the Spectroscopy Summary Sheets in addition to these over 100 min videos of solving NMR problems.
NMR and IR Spectroscopy Summary Sheets
1. How to Solve IR Problems in 3 steps!
2. NMR Spectroscopy – 5 pages that include
- Chemical Shift and Integration
- Number of NMR signals
- Spin-Spin Splitting
- 13C NMR Spectroscopy
- Determining the Structure of an Unknown















Thank you! These strategies are great and I feel more optimistic about NMR questions. Hopefully I won’t get them though… : )
I hope you got the NMR right on the test.
Super helpful for understanding NMR. Thank you!
Great to hear, Pokharel.
This was great! Thank you
You are welcome, Andrew.
Great! Thank You!