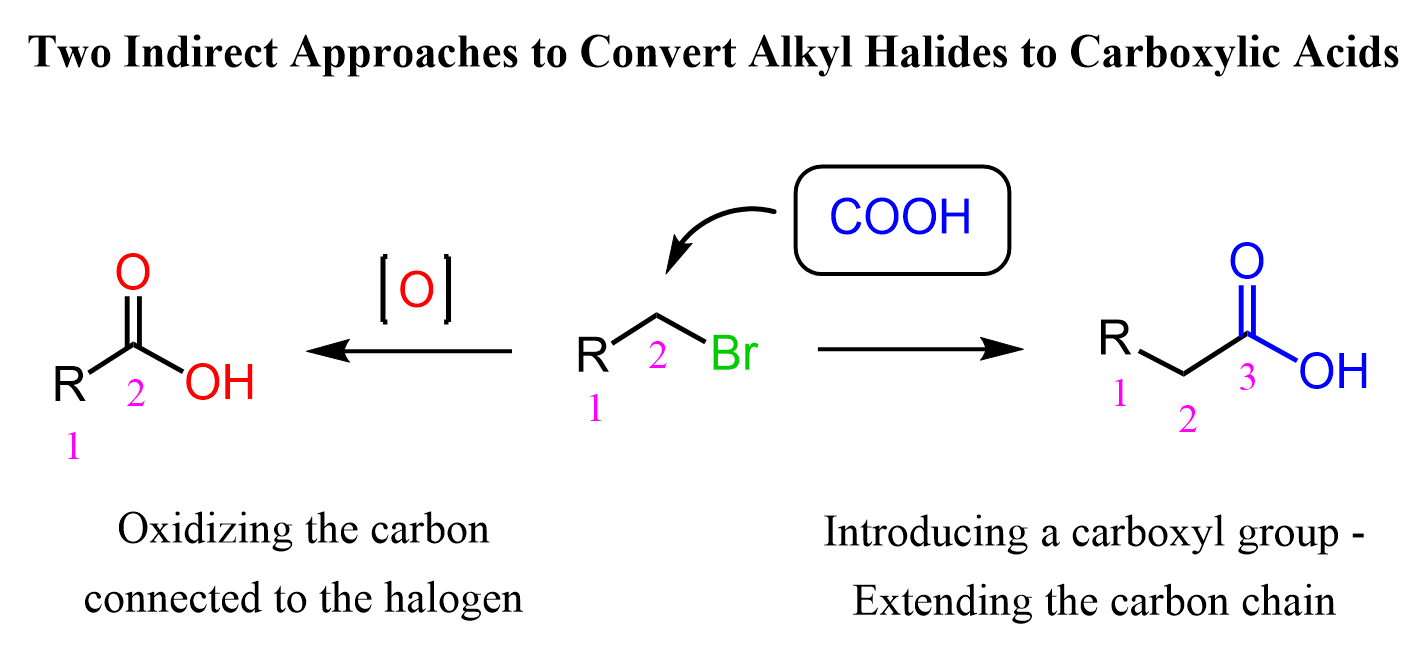
There are two main strategies we can use to convert alkyl halides to carboxylic acids: 1) Oxidizing the carbon connected to the halogen, 2) Introducing the COOH group, thus increasing the length of the carbon chain:
For the first approach, we can convert the alkyl halide to an alcohol, and then oxidize the alcohol to the corresponding carboxylic acid using a strong oxidizing agent such as chromic acid (the Jone’s oxidation), potassium permanganate (KMnO4) or sodium hypochlorite (NaClO – bleach):
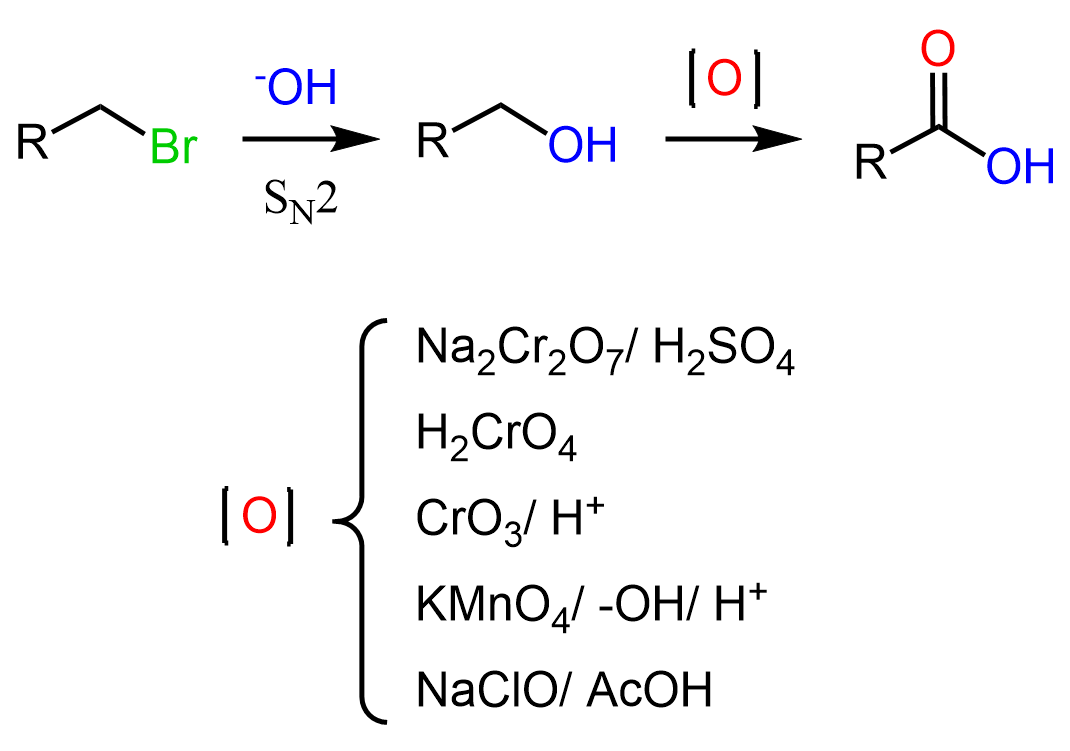
Remember, some reagents such as pyridinium chlorochromate (PCC), pyridinium dichromate (PDC), the Swern and Dess-Martin oxidation allow for selective oxidation of primary alcohols to aldehydes. The mechanistic details and selectivity of each oxidizing agent is covered in the Oxidation of Alcohols.
Notice also that we are using a primary alkyl halide because tertiary alcohols cannot be oxidized and the oxidation of secondary alcohols produces a ketone:

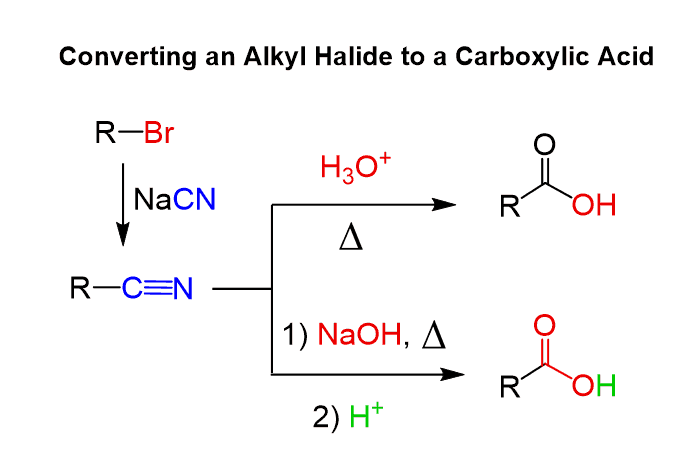
Let’s now consider the second approach which is incorporating a carboxylic acid to the starting alkyl halide. There is no direct way of doing this as we do not have a “COOH” or a “COO–“ block as a nucleophile. So, one way is to convert the alkyl halide to a nitrile via an SN2 substitution using for example NaCN in a polar aprotic solvent, and hydrolyze the nitrile under acidic or basic conditions:
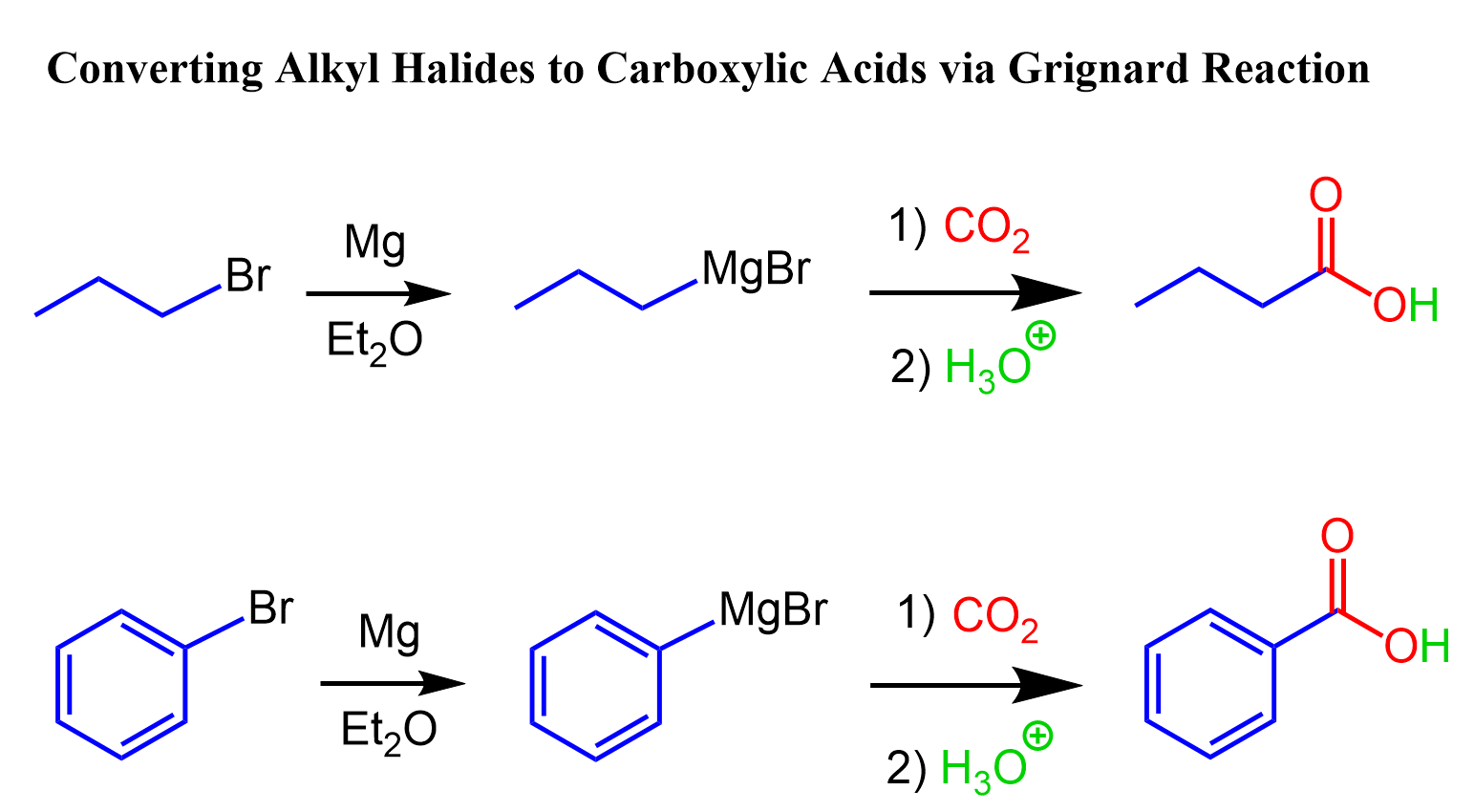
The second approach can be using the Grignard reaction. We can first convert the alkyl halide to a Grignard reagent and react it with carbon dioxide followed by an acidic workup:
Secondary Alkyl Halides to Carboxylic Acids
The Grignard reaction can also convert secondary and aryl (aromatic) halides to carboxylic acids.
We can also convert secondary alkyl halides to an alkene by Zaitsev or Hofmann elimination and oxidize the alkene to a carboxylic acid using ozonolysis or KMnO4:
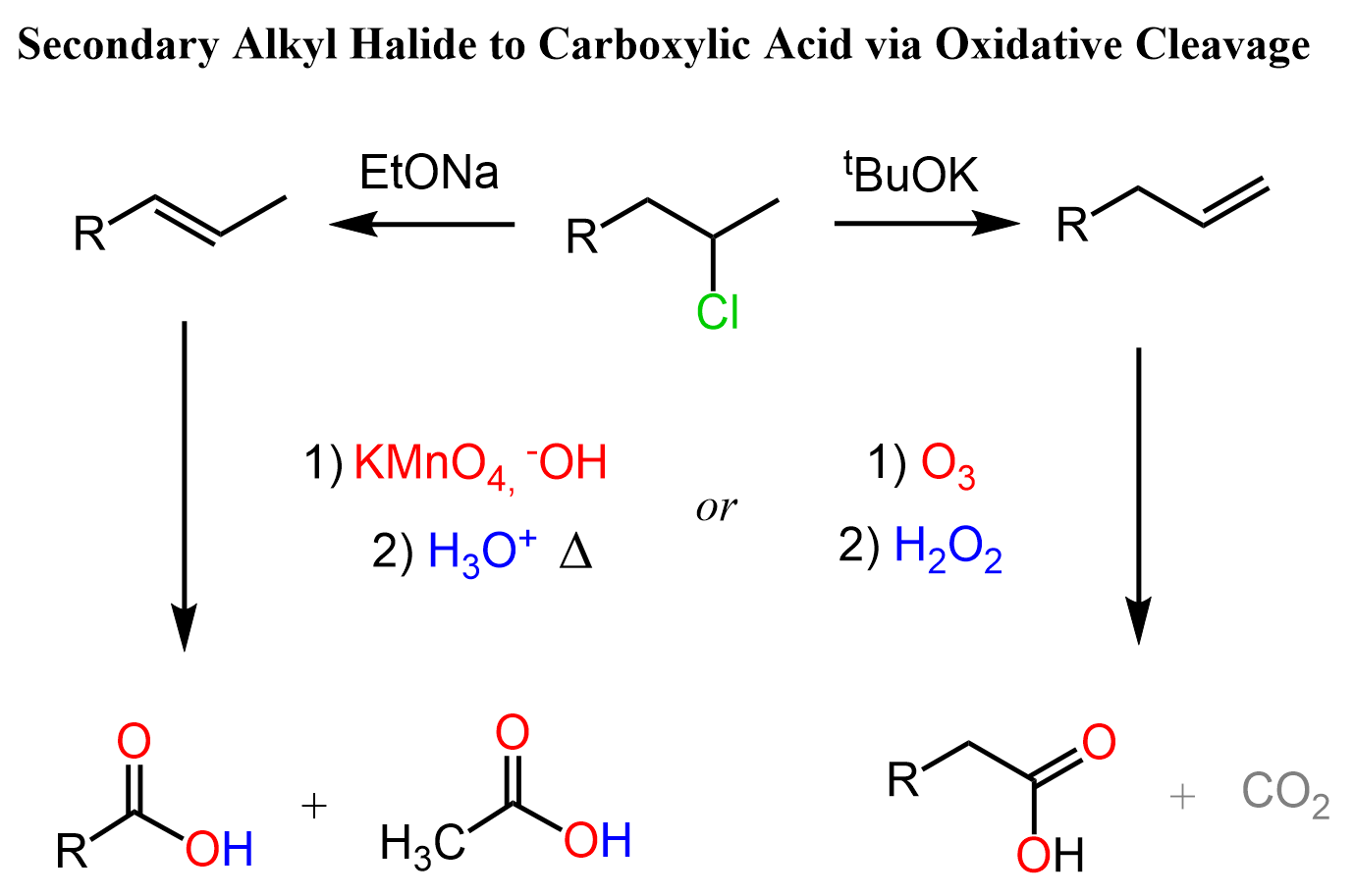
Notice that depending on the position of the halogen, we are losing at least one carbon so this may not be a practical approach to preparing a carboxylic acid, but it is something you may see in some synthesis problems.
If we need to keep the number of carbon atoms, we can move the position of the halogen by radical addition of HBr, then convert it to an alcohol and oxidize it to the corresponding acid as we have seen in the beginning of this discussion:
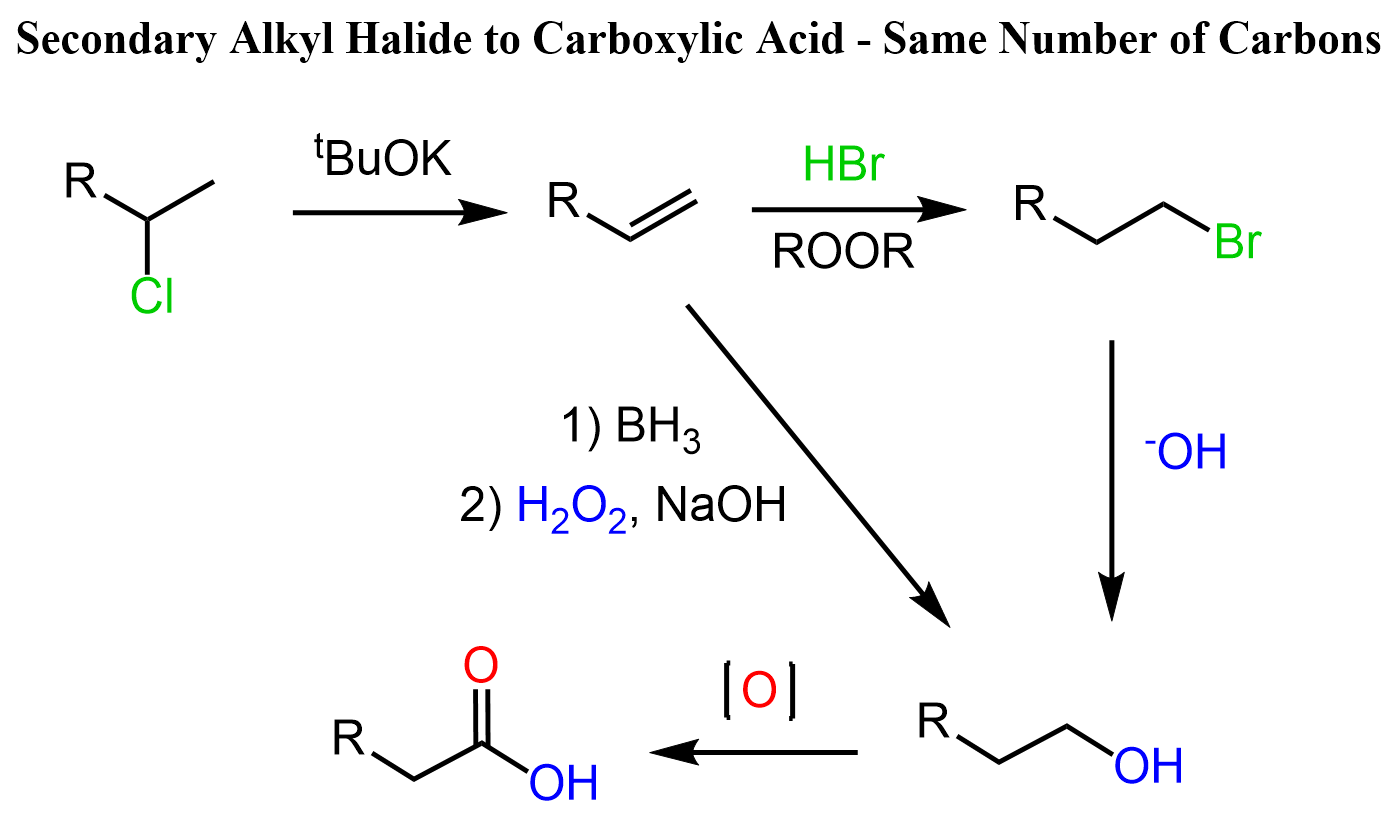
The shortcut above from the alkene to terminal alcohol is achieved by the hydroboration-oxidation of alkenes, which is an anti-Markovnikov hydration of the alkene.
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!

