When talking about Newman projections, we saw that the free rotation about single bonds changes the spatial arrangement of atoms in the molecule. As a result of these rotations, different conformations of molecules are possible. For example, two groups interchange between anti and syn conformation via a free rotation about a carbon-carbon single bond:
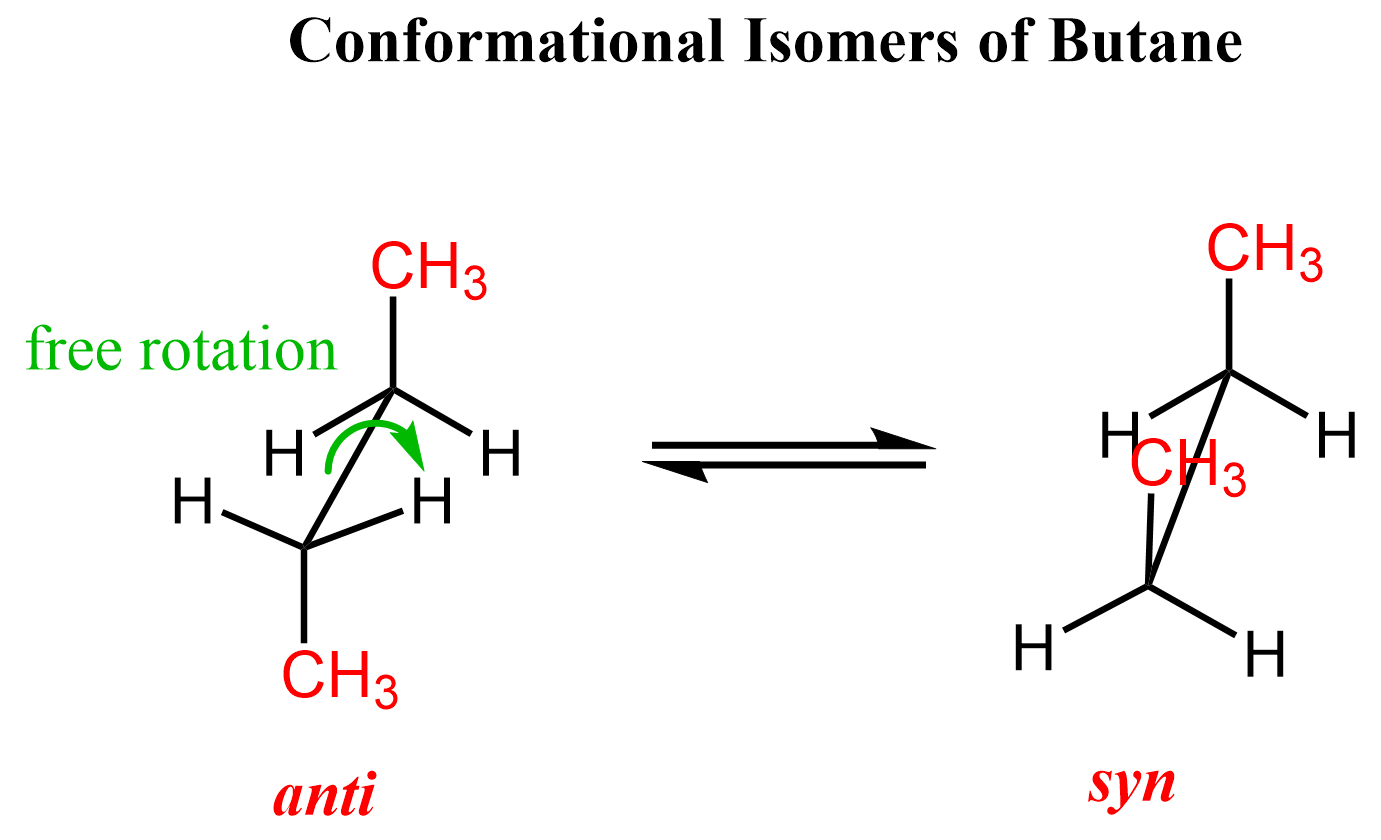
These two are conformational isomers of butane, which, again, are interconverted via free rotation about the central C-C single bond.
Now, the situation becomes different if the free rotation about single bonds is restricted. The most common and important such example is the arrangement of atoms in alkenes. We are talking specifically about the atoms connected to the double bond of the alkene.
For example, if we add a double bond between the central carbon atoms of butane by removing two of the hydrogen atoms, we will now have butene. In butene, just like in any other alkene, there is no rotation about the double bond. This means that if we have the methyl and hydrogen groups on the same or opposite sides of the double bond, we can’t interchange them anymore:

These two different spatial arrangements of atoms in butene or any other alkene make two distinct molecules called cis and trans isomers.
Notice that we are not talking about different connectivity of atoms – otherwise they would have been constitutional isomers. They are all connected the same, but have a different spatial arrangement due to restricted rotation.
In the example of but-2-ene, we highlighted the methyl groups, but this, of course, does not have to be any specific atom or group. We could have also focused on the spatial arrangement of hydrogens, or any other atoms/groups on the double bond, to label the molecule as cis or trans.
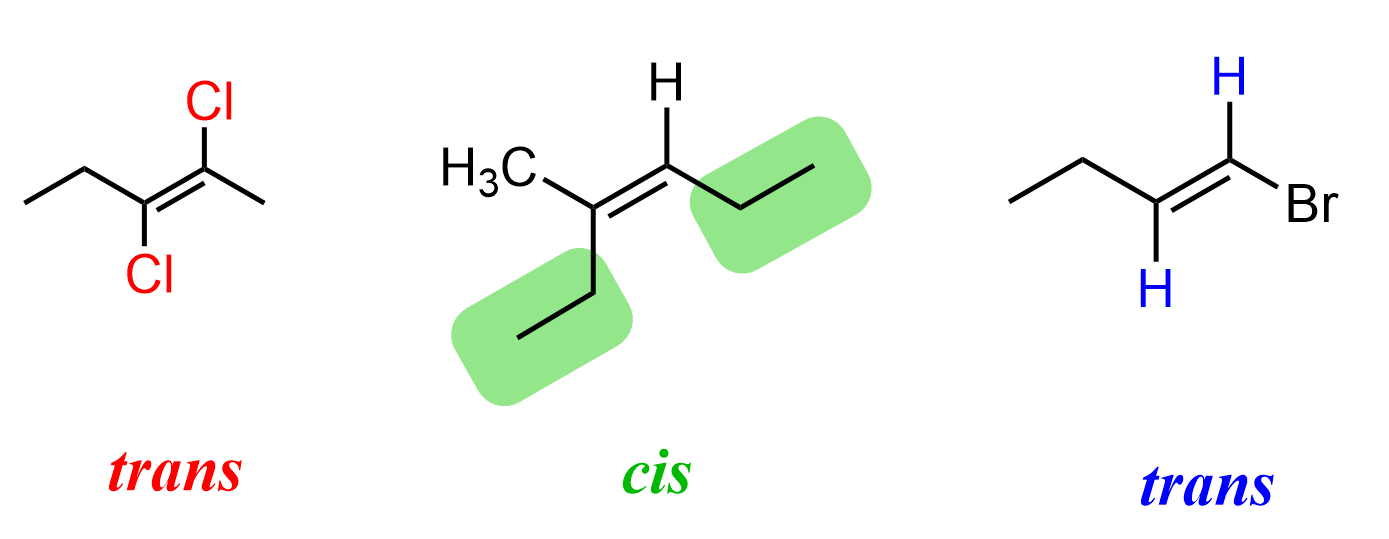
To summarize this definition, remember that when identical atoms on a double bond are on the same side, we have a cis alkene, and when they are on opposite sides of the double bond, we have a trans alkene.
Cis and Trans, Geometric and Stereoisomers
Cis and trans isomers are also called geometric isomers, which are a type of stereoisomer. Remember from the chapter on stereochemistry that stereoisomers are compounds with the same molecular formula and the same connectivity of atoms but differ in the spatial arrangement of their atoms. This means cis and trans (geometric) isomers fall under the category of stereoisomers, and, if we want to specify further, they are diastereomers because they are not nonsuperimposable mirror images of one another.

Just like R and S configurations, the cis and trans configurations cannot be changed without breaking bonds. Therefore, both of these are configurational isomers within the category of stereoisomers. The terminology can feel overwhelming at times, and you can refer to this post for an overview and a comprehensive chart of isomerism, but for your organic chemistry class, remember: cis and trans are geometric isomers, and they are a type of diastereomer.
Do All Alkenes Have Cis and Trans Isomerism?
Now, in order to have cis and trans isomers, the alkene must have two different atoms or groups connected to each carbon of the double bond. For example, the following two alkenes cannot have cis–trans isomerism because one of the double-bonded carbons bears two identical groups.
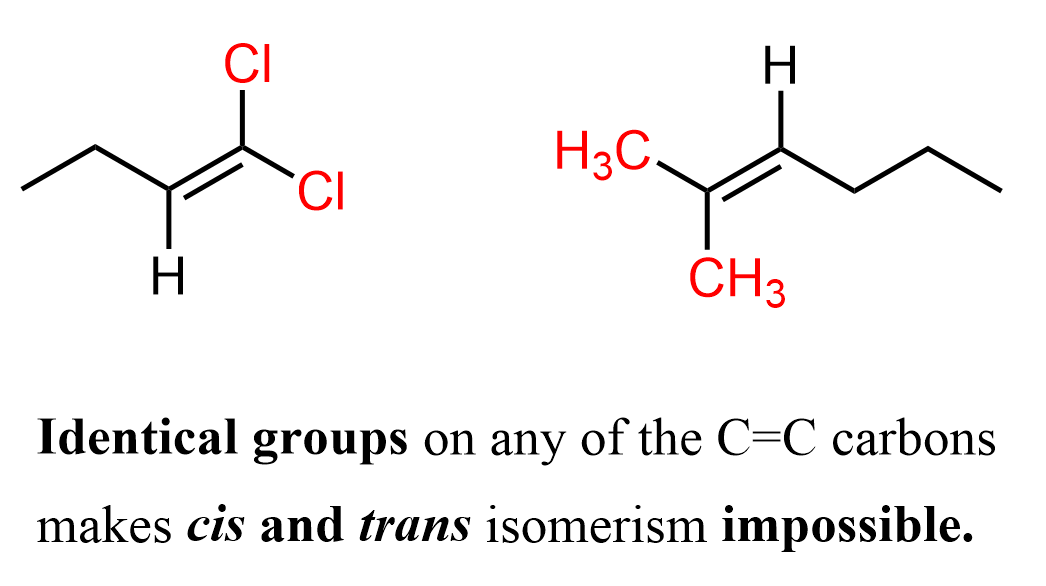
Notice that we are talking about identical atoms or groups on the same carbon atom of the double bond. Cis and trans isomerism is only possible if both carbons in the C=C have two different atoms or groups attached. If either carbon has two identical atoms or groups, the arrangement on the other carbon no longer matters – the alkene cannot have cis/trans isomers.
Cis and Trans Isomerism in Cycloalkanes
Although cycloalkanes look to have nothing in common with alkenes, one feature that unites them is the restricted rotation about carbon–carbon bonds.
While in alkenes the groups on the double bond are just stuck in the same plane, there is still some flexibility in cycloalkanes. Recall the different conformations of cycloalkanes such as chair, half-chair, envelope, etc..
This, however, does not allow two groups to switch their relative orientation. For example, if two methyl groups are pointing up and down in one chair conformation, they stay like this in the other chair conformation too, which is formed via a ring-flip:
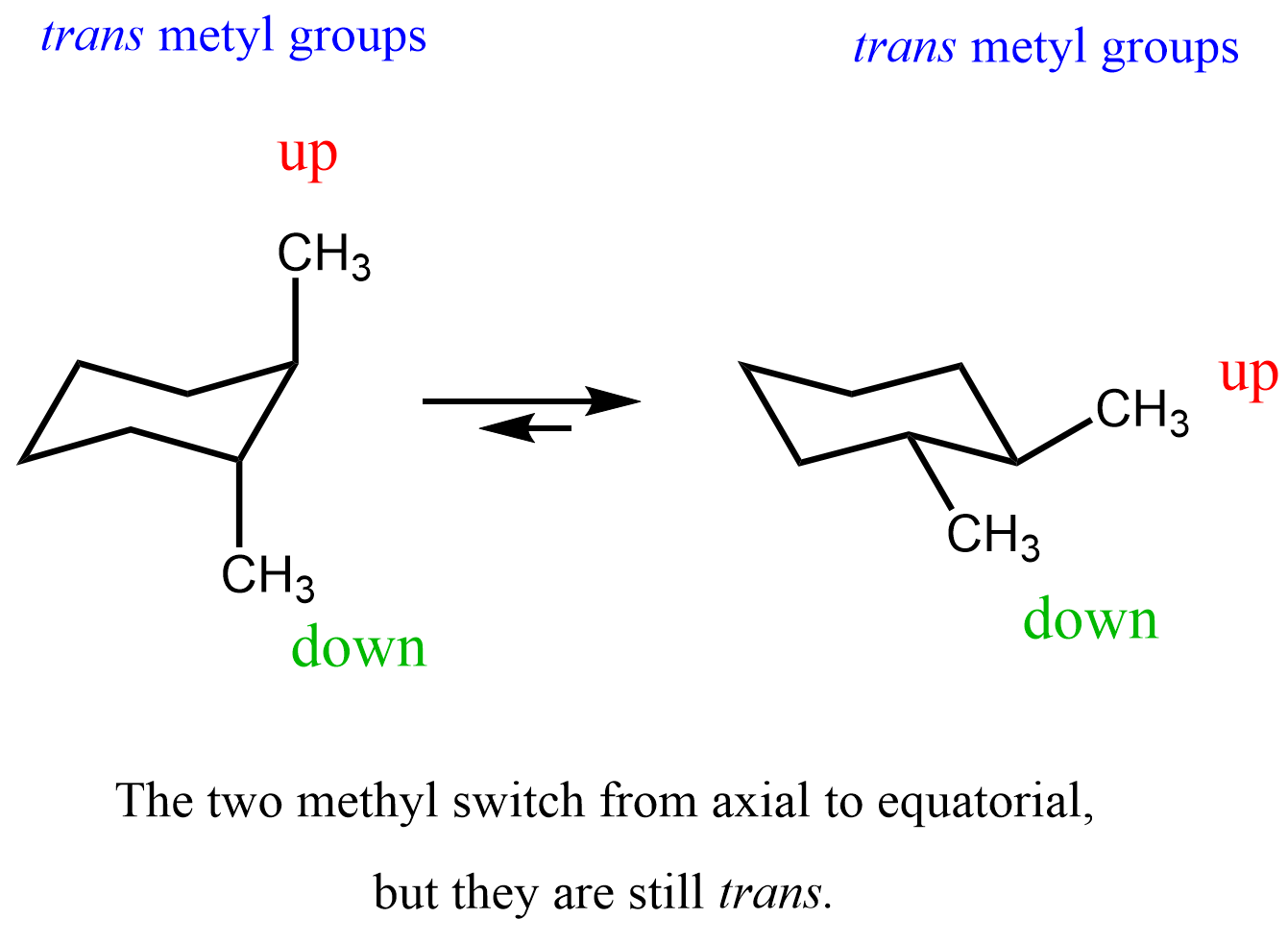
Once again, the two methyl groups switched from axial to equatorial positions; however, their relative orientation is trans.
Yes, we use the cis and trans notation for cycloalkanes. Compare the following 1,2-dimethylcyclohexane to the previous one. The two methyl groups are now pointing in the same direction, and it does not matter if they are axial or equatorial.
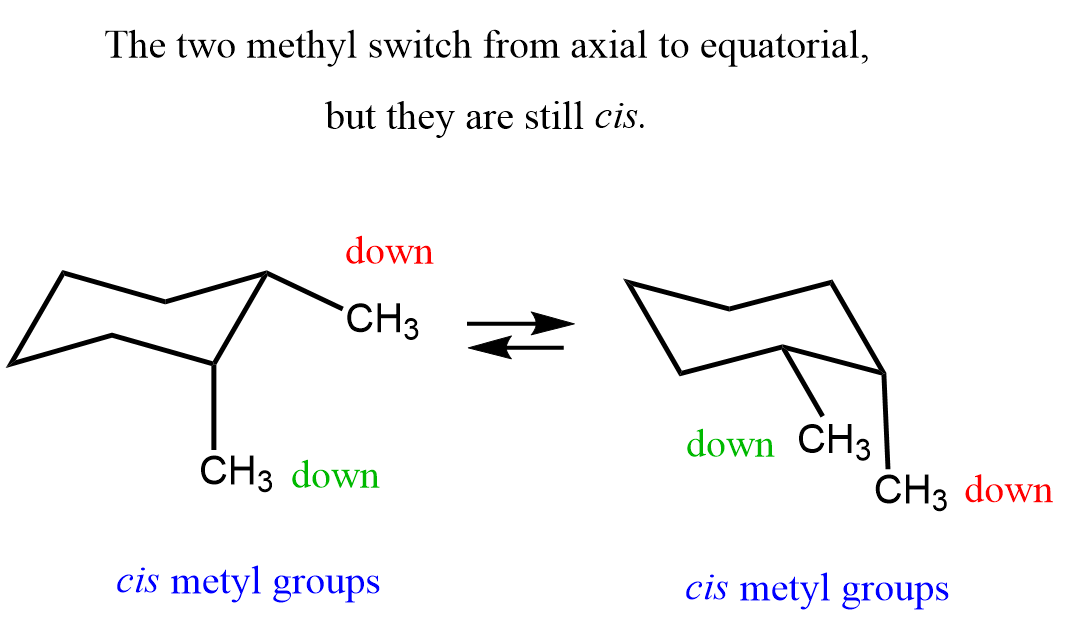
This is the cis isomer of 1,2-dimethylcyclohexane, and both configurations can also be represented using bond-line drawings for an alternative perspective.

Like in the case of alkenes, the cis and trans are used even if the two groups on the ring are not identical:

To make it easier, draw the hydrogen atoms on the carbons and determine the configuration based on their relative orientation. If both hydrogens are on the same side, it is cis, and if they are on opposite sides, then we have a trans isomer.

Cis and Trans in IUPAC Nomenclature
Although we often use cis and trans in the names of alkenes, especially in simpler cases, the preferred IUPAC nomenclature uses the E/Z system. This is because the cis/trans naming only works when each carbon of the double bond has one identical group for direct comparison.
For example, how can we classify this alkene as cis or trans if all four groups on the double bond are different:
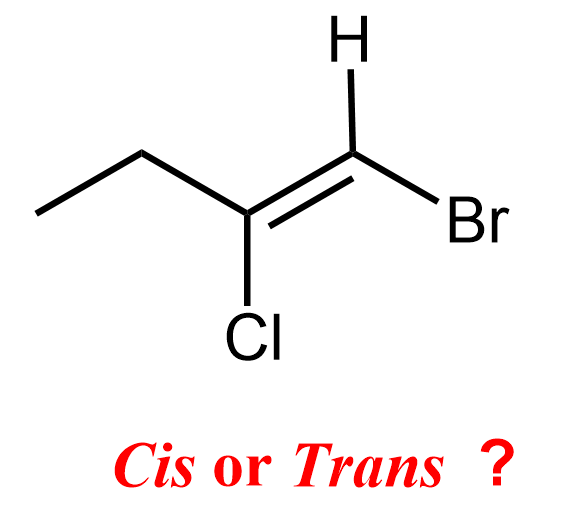
In complex alkenes like this, we use the E/Z notation, which is based on Cahn-Ingold-Prelog priority rules, and provides an unambiguous way to describe the geometry.
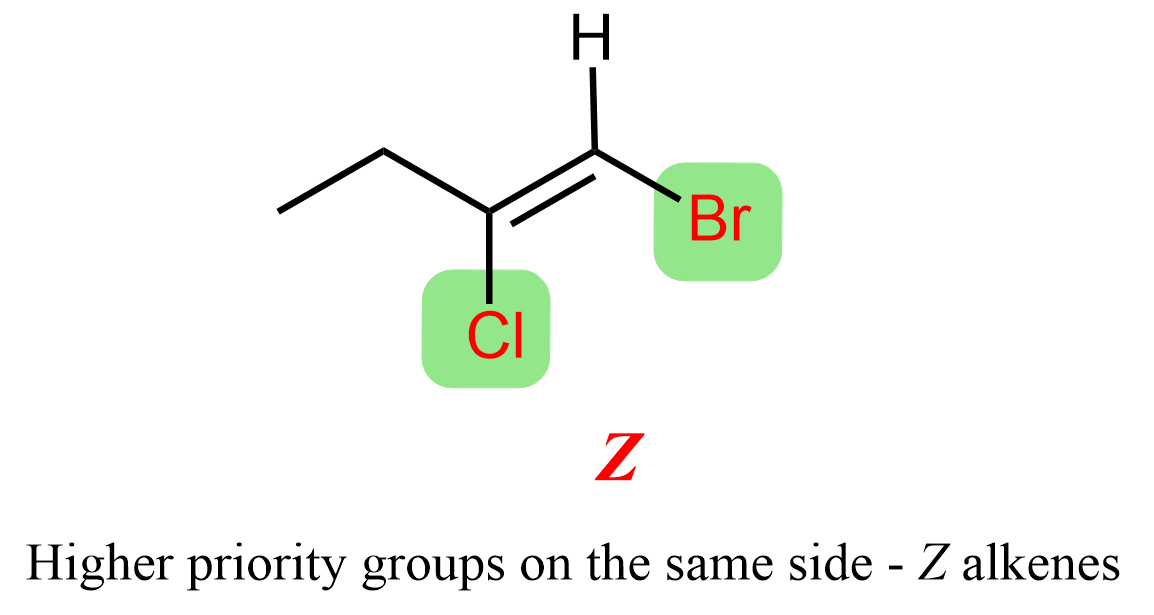
We assign priorities to the two groups on each carbon using the same rules as for R and S configurations. If the higher priority groups are on the same side of the double bond, the alkene is assigned the Z configuration. If they are on opposite sides, the configuration is E.

In this case, for the alkene shown above, there is a bromine and a hydrogen atom on carbon 1, while on carbon 2, there is a chlorine atom and an ethyl group. Bromine has a higher atomic number than hydrogen, so bromine gets the higher priority on carbon 1. On carbon 2, chlorine has a higher priority than the ethyl group because chlorine has a higher atomic number than carbon. Since the higher priority groups, bromine on carbon 1 and chlorine on carbon 2, are positioned on the same side of the double bond, the molecule is assigned the Z configuration.
Here is an example of an alkene with an E configuration:
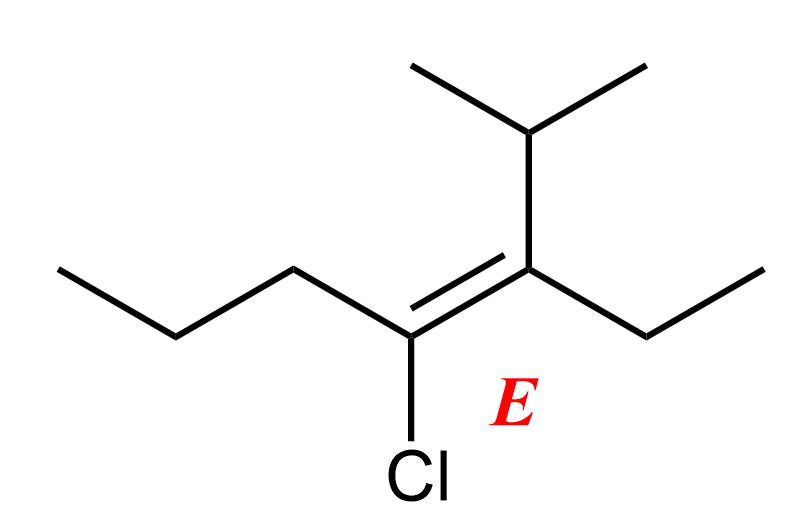
Let’s see why the configuration is E.
First, we need to identify the two groups attached to each of these carbons.
On carbon 3, we have an ethyl and an isopropyl group. Isopropyl has higher priority because it has more carbon branches. On carbon 4, there’s a chlorine and a propyl group, and chlorine wins due to its higher atomic number. Since the higher-priority groups are on opposite sides of the double bond, the configuration is E.

Therefore, the IUPAC name is (E)-4-chloro-3-ethyl-2-methylhept-3-ene. We have a separate post on the IUPAC nomenclature of alkenes, so check that out for more details and examples on naming alkenes.
Check this article on E and Z configuration of alkenes for more details and examples on this topic.
Summary of Cis and Trans Isomerism
Cis and trans isomers are a specific type of stereoisomer known as geometric isomers, distinguished by the relative positions of identical or similar groups around a double bond or within a ring. These isomers cannot interconvert without breaking bonds, making them configurational isomers. While cis and trans notation works well for simple cases where each carbon of the double bond has one identical substituent, it becomes insufficient for more complex molecules. That’s why the IUPAC system uses the more universal E/Z notation, which relies on priority rules to clearly and unambiguously describe the spatial arrangement of substituents around double bonds, regardless of complexity.



