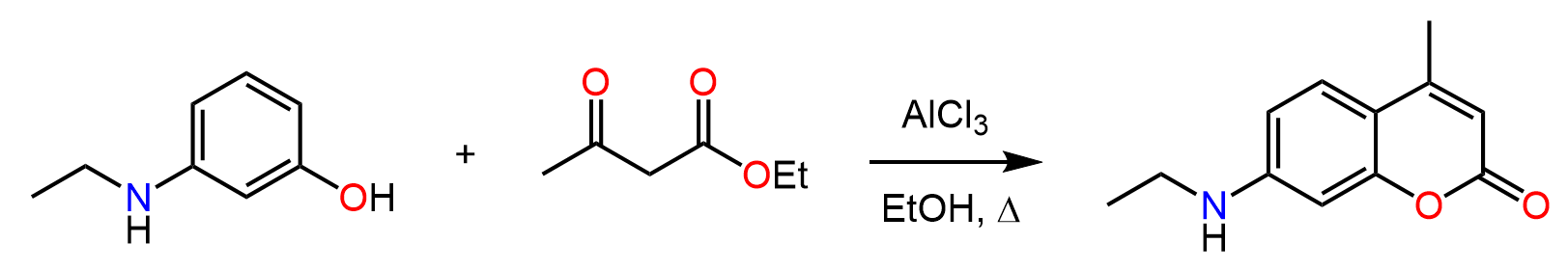
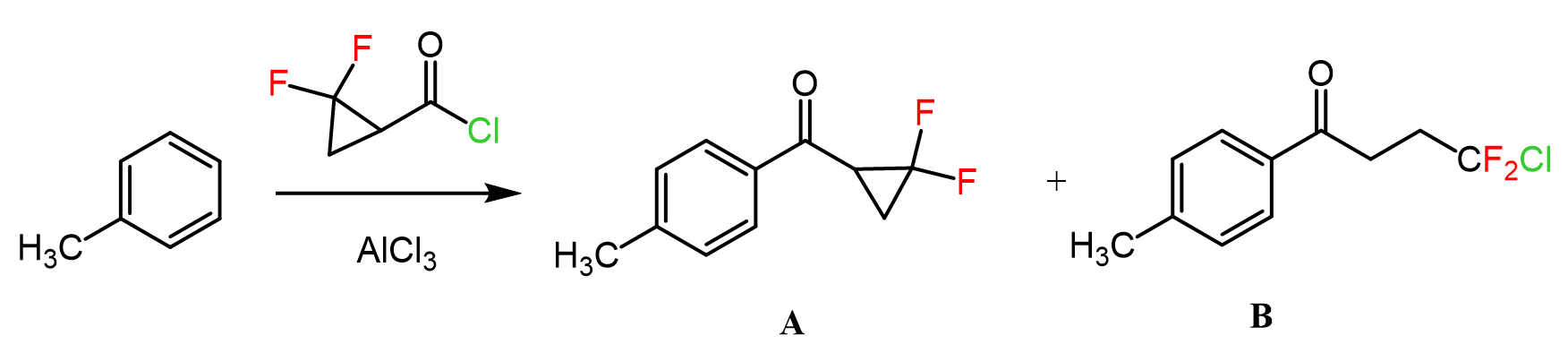
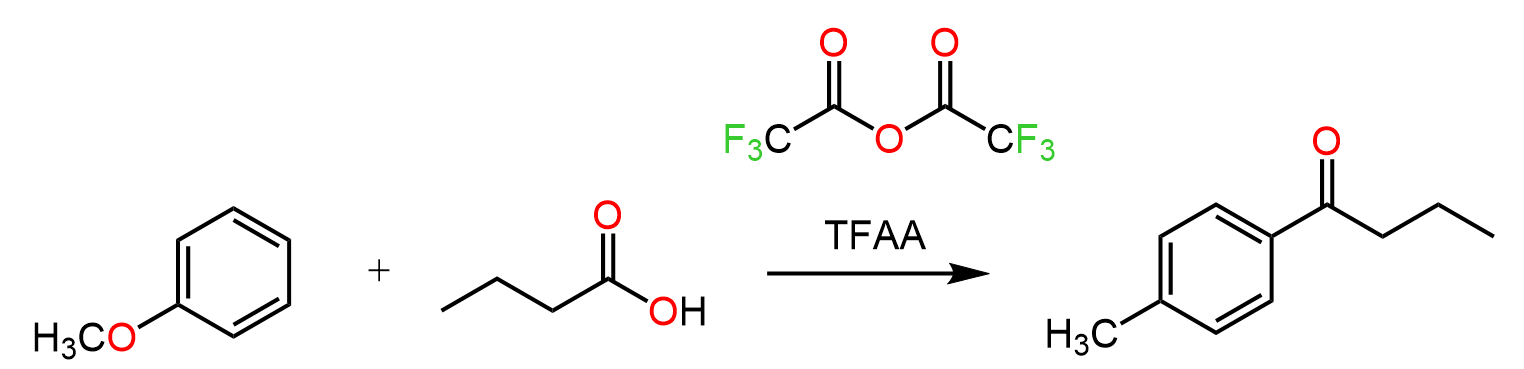
In the previous post, we talked about Friedel-Crafts alkylation – a classic reaction for attaching alkyl groups to benzene rings. A similar reaction is used for adding an acyl group to the aromatic ring, thus making a ketone. This is called Friedel-Crafts acylation, and just like in the case of alkylation, it is carried out in the presence of a Lewis acid catalyst, such as AlCl₃.
Let’s put together a general scheme together with examples of and discuss the mechanism and some details of the Friedel-Crafts acylation:
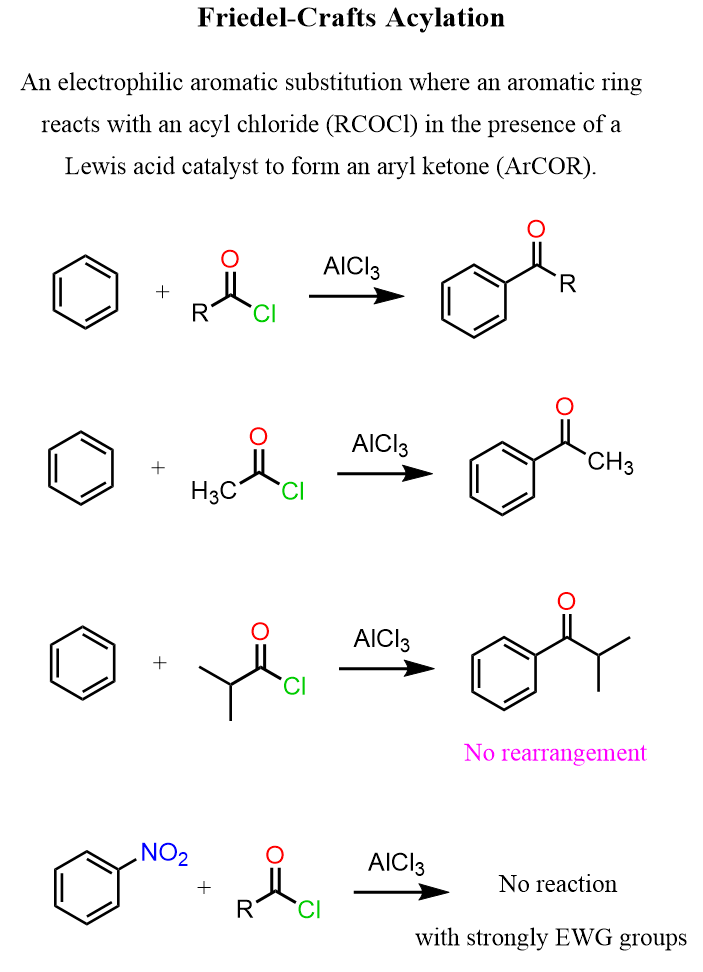
The Mechanism of Friedel-Crafts Acylation
Like in other EAS reactions we have seen, such as the halogenation, nitration, and sulfonation, in the Friedel-Crafts acylation too, there is an electrophile that is attacked by the aromatic ring, which acts as a nucleophile due to its π electrons.
The key electrophile in this reaction is the acylium ion (R–C≡O⁺). This ion is generated when AlCl₃ pulls chloride away from the acyl chloride, creating a resonance-stabilized carbocation that’s ready to be attacked by the π electrons of the aromatic ring.
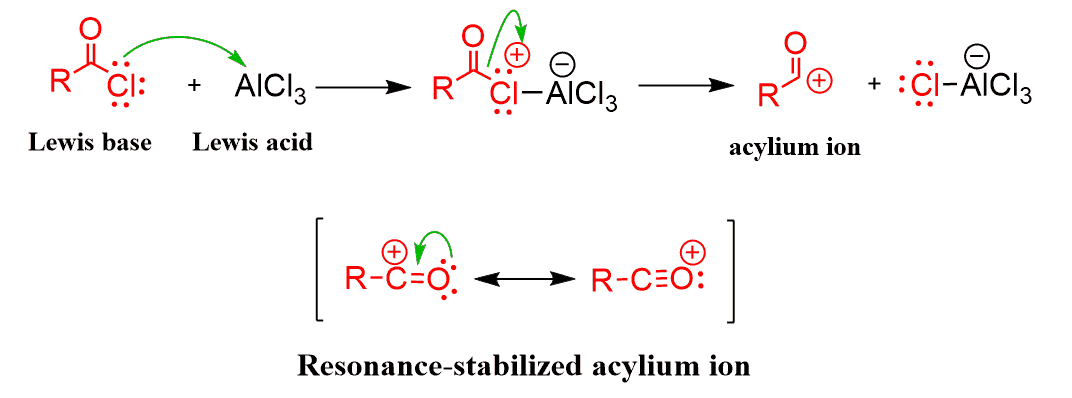
Once the aromatic ring attacks the electrophile, the reaction proceeds through the familiar arenium ion (σ-complex) intermediate. A proton is then lost, restoring aromaticity and yielding a ketone group on the ring.
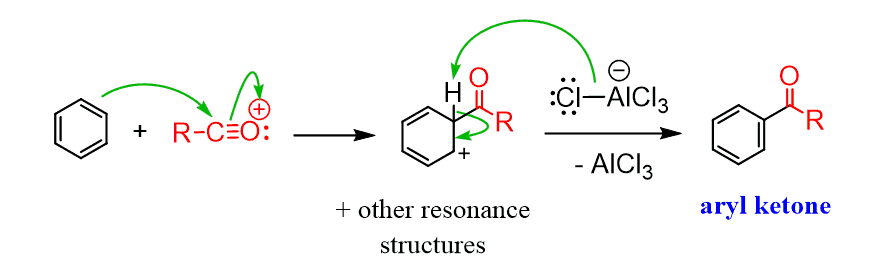
Advantages of Friedel-Crafts Acylation
You may wonder why we can’t do a Friedel-Crafts alkylation and then convert the product into an aromatic ketone by a subsequent functionalization of the benzylic position. There are some key advantages to using the direct acylation of the aromatic ring:
- No rearrangements.
The acylium ion is stable and doesn’t scramble like alkyl carbocations often do. That means you get exactly what you expect.

Recall that the alkyl carbocation formed in Friedel–Crafts alkylation reactions may undergo rearrangements, just like we’ve seen in SN1 and E1 reactions.
Therefore, Friedel-Crafts acylation allows for easier access to primary-alkyl aromatic compounds when the possibility of rearrangement is present. Once you’ve installed the acyl group, you can reduce the ketone (using Wolff–Kishner or Clemmensen reduction), effectively giving you a primary alkyl group without worrying about carbocation rearrangement.

- Monoacylation.
The carbonyl group (C=O) on the product actually deactivates the ring, making it less reactive. So, unlike alkylation, you don’t get a bunch of unwanted extra substitutions.
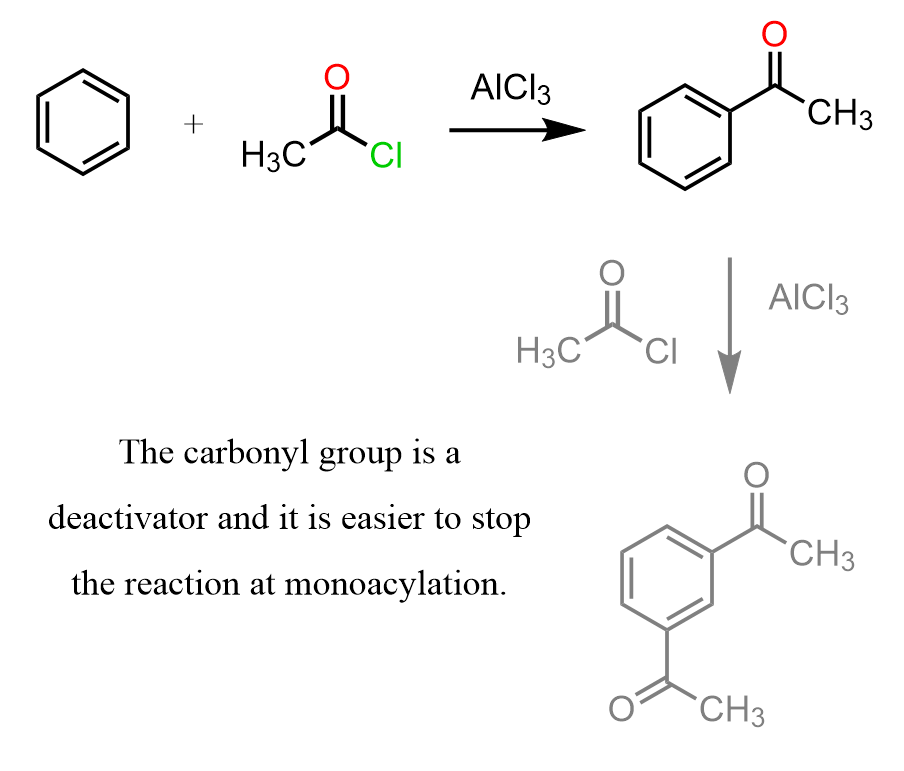
Once again, remember that alkyl groups are activators; therefore, in Friedel-Crafts alkylation, once the first alkyl group is added, it makes the ring more reactive, and it is difficult to stop the reaction at monoalkylation.
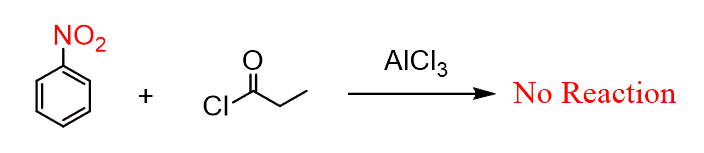
In general, keep in mind that Friedel-Crafts reactions are the slowest among electrophilic aromatic substitutions, and they do not work when a strong deactivator such as NO2, CF3, and SO3H is present on the ring.
Check out this article for more limitations and exceptions in electrophilic aromatic substitutions.
Intramolecular Friedel-Crafts Acylation
Friedel–Crafts acylation can also be done intramolecularly, especially if you have an aromatic ring and an acyl chloride group within the same molecule. This is a popular method to form five- or six-membered rings in synthesis.

Can We Introduce an Aldehyde by Friedel-Crafts acylation?
Can we prepare an aryl aldehyde instead of an aryl ketone? The answer is no, we cannot directly convert an aromatic ring into an aldehyde using Friedel-Crafts acylation. The problem is that formyl chloride and formyl anhydride are not stable reagents, so we can’t use them practically.

That’s why other reactions like the Vilsmeier-Haack or Gattermann–Koch reactions are used to install aldehyde groups on aromatic rings.
Summarizing Friedel-Crafts Acylation
Friedel–Crafts acylation is one of the most reliable ways to introduce a ketone into an aromatic ring. It avoids the issues of polyalkylation and rearrangements that need to be considered in Friedel-Crafts alkylation reactions.