This is a 108-question, multiple-choice quiz on stereochemistry.
The stereochemistry quiz comes with a 3-hour video solution showing a detailed step-by-step solving of all the questions. It’s a must-watch, master course video!
These are the topics and some example questions from the quiz.
Chirality and Enantiomers
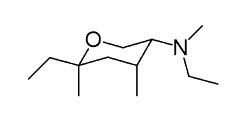
How many chiral centers does the following compound have?
Enantiomers, Diastereomers, the Same or Constitutional Isomers
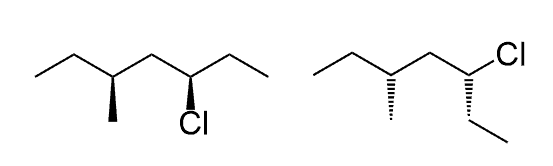
What is the relationship between these two structures?
The R and S configuration
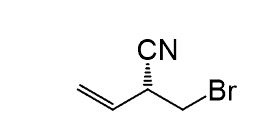
Identify all the chiral centers and determine the absolute configuration as R or S:
Optical Activity
Enantiomeric Excess (ee): Percentage of Enantiomers from Specific Rotation with Practice Problems
How many percent of cholesterol and its enantiomer are present in a sample with an observed specific rotation of -22.4°? The specific rotation of pure cholesterol is -32°.
A) 85% cholesterol and 15% enantiomer
B) 15% cholesterol and 85% enantiomer
C) 70% cholesterol and 30% enantiomer
D) 30% cholesterol and 30% enantiomer
Symmetry and Chirality. Meso Compounds
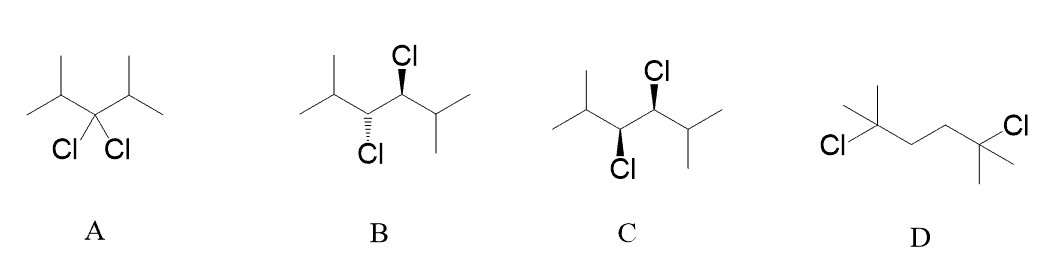
Which of the following is a meso compound?
Fischer Projections
What is the relationship between these two structures?
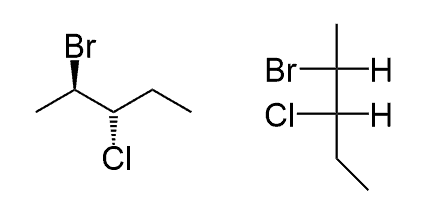

How do I know what the actual answer is ?
Hi Jessica,
The correct answer is highlighted in green and your answer, in case it is incorrect, is marked in red.
If both R and S character are flipped with enantiomers, how is C the correct answer? Wouldn’t C be the superimposable mirror image?
That is regarding question #12
Hi Lorin,
You are correct about the concept of switching the configuration of all the chiral centers in enantiomers.
However, C is in fact a non-superimposable mirror image of the given molecule. Simply imagine placing a mirror to the right side of the molecule-you will get C.
B, on the other hand, is the same compound flipped by 180o.
Remember, when in dpoubt, you can always detemine the configuration of the chiral centers and compare them.