Aldehydes and ketones react with water in the presence of an acid or a base, forming a Hydrate – Geminal Diol:

The addition of water to most aldehydes and ketones is unfavorable, and the equilibrium is shifted mainly toward the carbonyl compound.
A few exceptions are the aldehydes with nearby electron-withdrawing groups and the simplest aldehyde, formaldehyde, which exists primarily as the gem-diol in aqueous solution:

Let’s understand this behavior of aldehydes and ketones.
In general, the reactivity of the carbonyl compound (or any compound for that matter) depends on its stability. The more stable, the less reactive.
Now, the efficiency of the addition reactions to an aldehyde or a ketone is determined by how electrophilic the C=O carbon atom is.
The partial positive charge of the carbon is suppressed by attached alkyl groups (remember, alkyl groups are electron donors), and therefore, aldehydes are more reactive than ketones:
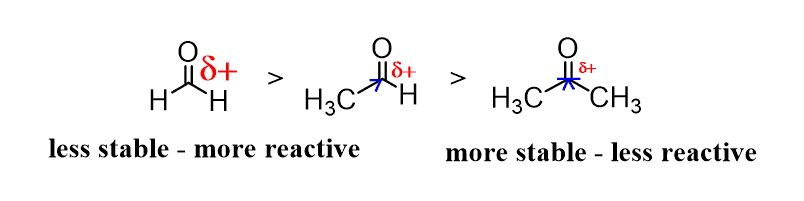
Consequently, electron-withdrawing groups near the carbonyl carbon decrease its electron density, thus making it more reactive in nucleophilic addition reactions.
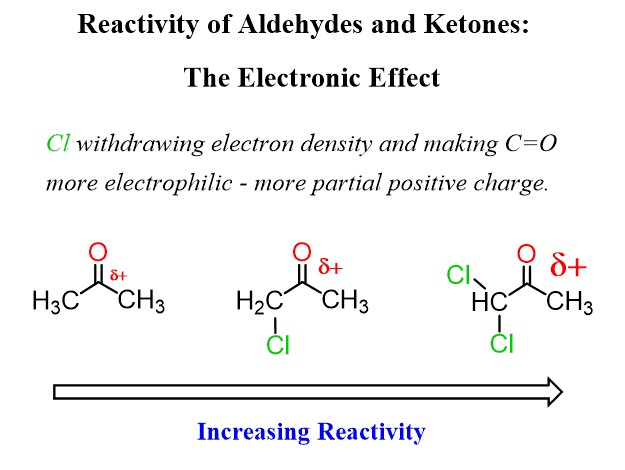
This was the electronic effect, but there is also the Steric Effect.
The tetrahedral product of the addition reaction is less crowded/bulky/sterically hindered and hence more stable in the case of aldehydes:

Also, bulkier alkyl groups make the nucleophilic attack more difficult/slower.
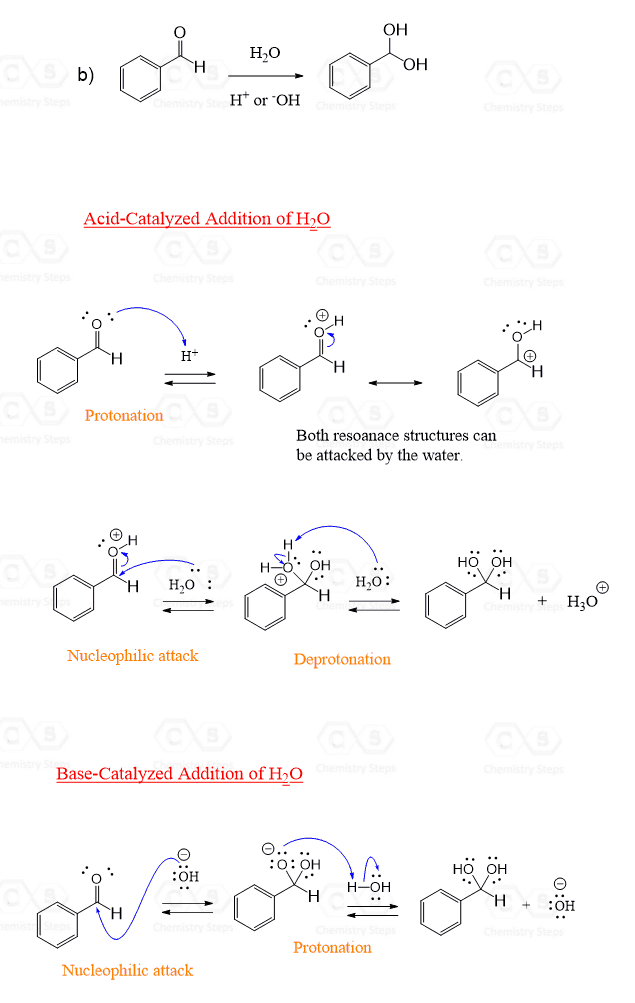
Aldehyde and Ketone Hydration Mechanism
Before discussing the acid and base-catalyzed hydration of aldehydes and ketones, it is worth mentioning that the water itself reacts slowly with the carbonyl group, and the acid and base are only used to speed up the process.
The mechanisms are slightly different.
In the base-catalyzed hydration, the nucleophile is –OH since it is a stronger nucleophile than water.
So, in the first step, the –OH attacks the carbonyl carbon, cleaving the π bond, and moving an electron pair onto oxygen. This forms a negatively charged intermediate which, upon protonation by water, results in a geminal diol:

The acid-catalyzed addition, on the other hand, starts with a protonation of the carbonyl oxygen. This makes the carbonyl more reactive by decreasing the electron density.
The Lewis acid activation of carbonyl compounds is a common scheme that we have also seen in the hydride reduction of carbonyl compounds by LiAlH4, NaBH4, etc.
Once the protonation happens, water now acts as a nucleophile, attacking the carbonyl group:

To summarize the mechanisms, the order of nucleophilic attack and protonation is reversed in acid and base-catalyzed hydration of aldehydes and ketones. Also, the water and hydroxide ion serve as a nucleophile in each reaction, respectively.

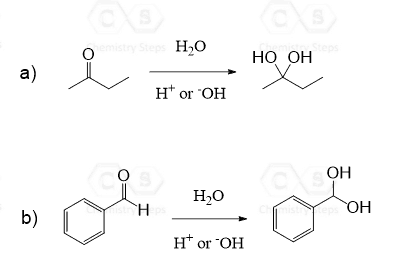
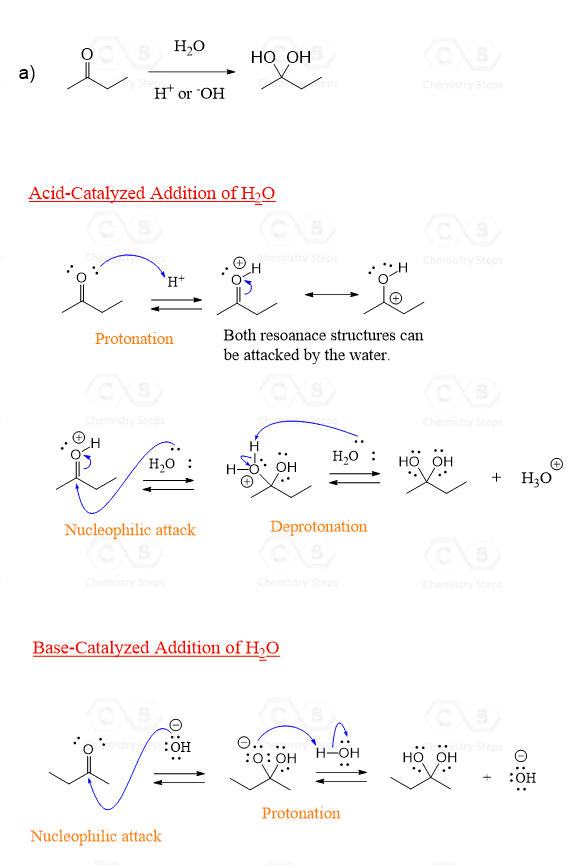
The site was very helpful I appreciate you
Thank you