In the previous post, when discussing the mechanism of radical halogenation, we saw that polyhalogenation is a common issue as it results in a mixture of haloalkanes. Monohalogenation, on the other hand, is more useful for preparing haloalkanes used in organic synthesis. It can be achieved by a selective halogenation using bromine.
So, the two main questions we will address in this post:
What does it mean by a selective halogenation, and why bromine but not chlorine?
First, by selectivity, we are referring to the regioselectivity of the radical halogenation.
For example, can we mix propane with a halogen and selectively substitute the hydrogen on carbon 1 or 2 while leaving the other positions intact?
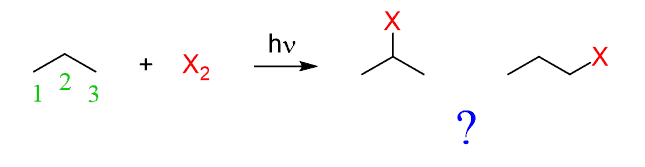
The answer is yes, it is possible, but it only works effectively when bromine is used.
For example, when propane is mixed with an equimolar amount of chlorine or bromine, a mixture of constitutional isomers is obtained in the following ratio:

Halogenation of the secondary carbon is the main path in both reactions.
This preference for halogenation of the secondary carbon is explained by the higher stability of more substituted radicals. They follow the same pattern as carbocations since in both cases we have an sp2-hybridized carbon lacking an octet:

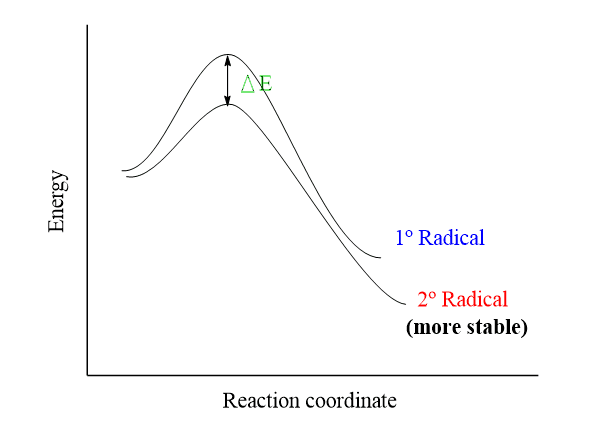
The more substituted radicals are more stable, so a tertiary radical is more stable than a secondary radical, which is more stable than a primary radical.
Recall also that the rate-determining step of the radical halogenation is the first propagation step. Therefore, the stabilities of the secondary and primary radicals of propane determine on which carbon the halogenation is going to occur.
This can be seen on the energy diagram, demonstrating the formation of primary and secondary radicals. The more stable the radical, the more easily it is formed:
Another way of estimating the stabilities of radicals is by comparing the energies of homolytic bond dissociation for primary and secondary C-H bonds.
Below are shown the values of DH ° for the primary and secondary C−H bonds of propane:
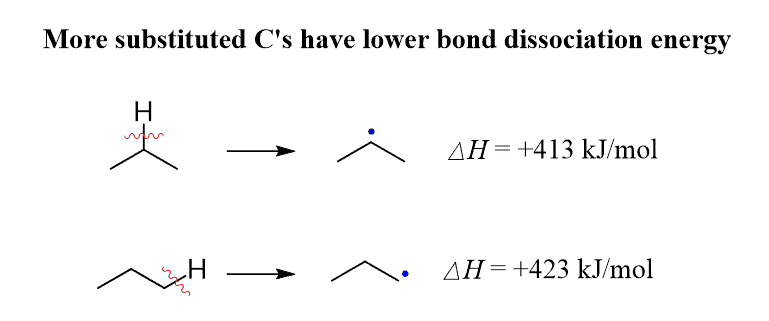
This explains why the secondary position of propane is more reactive. However, it does not address the better selectivity of the bromination over chlorination when comparing the ratio of the primary and secondary alkyl halides formed in the halogenation of propane.
There is one important difference in the radical bromination and chlorination reactions that explains this advantage of bromination.
The thing is that the rate-determining step of chlorination is exothermic, while the rate-determining step of bromination is endothermic:
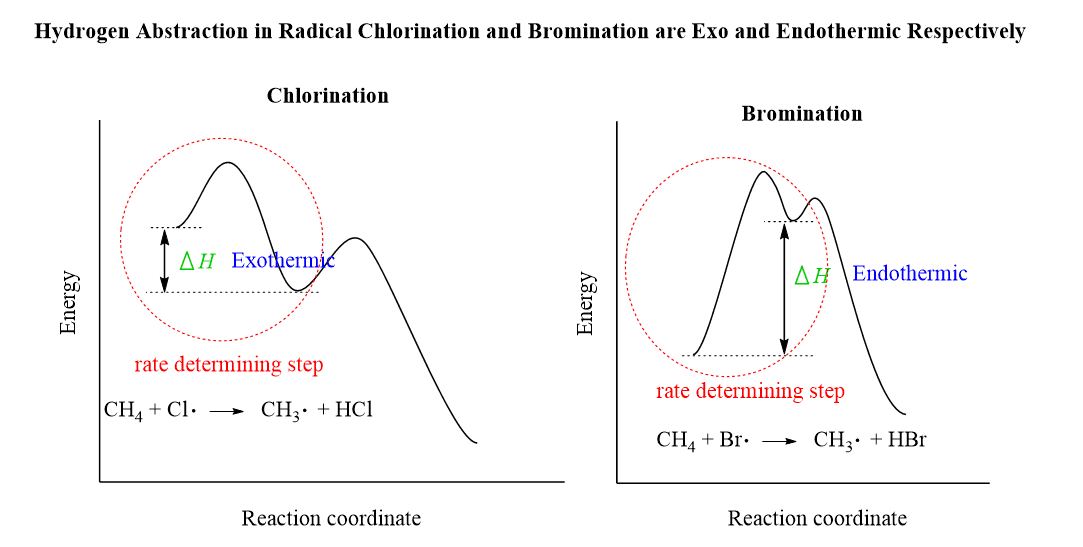
This difference means that the activation energy of the bromination is higher than the chlorination according to the Hammond postulate:

A reminder about the Hammond postulate: the structure of the transition state resembles the structure that appears closer to it on the energy diagram.
Therefore,
- Transition states in endothermic reactions resemble the products.
- Transition states in exothermic reactions resemble the starting materials.
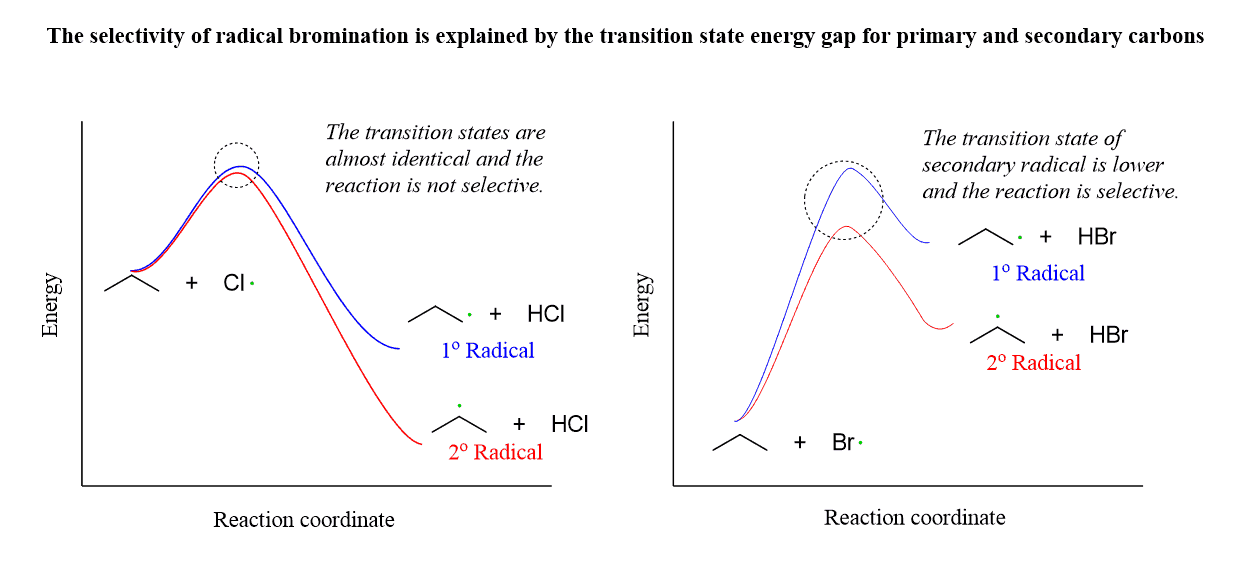
And this means that the stability of the product (in our case, the haloalkanes) greatly affects the stability of the transition state in the bromination reaction, but not in the chlorination. Again, this is because the bromination is endothermic, closer to the transition state.
The gap of the activation energy for the bromination of a secondary and a primary carbon is bigger, which is the source of the higher regioselectivity:
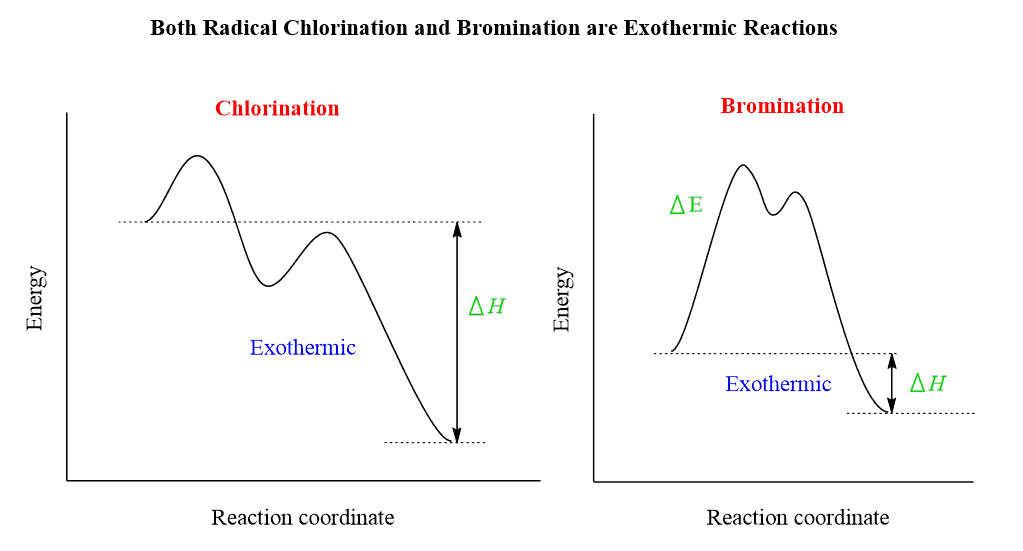
Notice that we are not talking about the overall reactions for which they are both exothermic:
Statistical Distribution and Selectivity of Radical Halogenation
There was one important factor we did not consider when comparing the ratio of products in the halogenation of primary and secondary carbons in propane. And that is the number of hydrogens connected to the primary and secondary carbon atoms.
Propane has two methyl groups and thus six primary hydrogens that can react with a given radical of halogen, and only two secondary hydrogens available for this reaction:
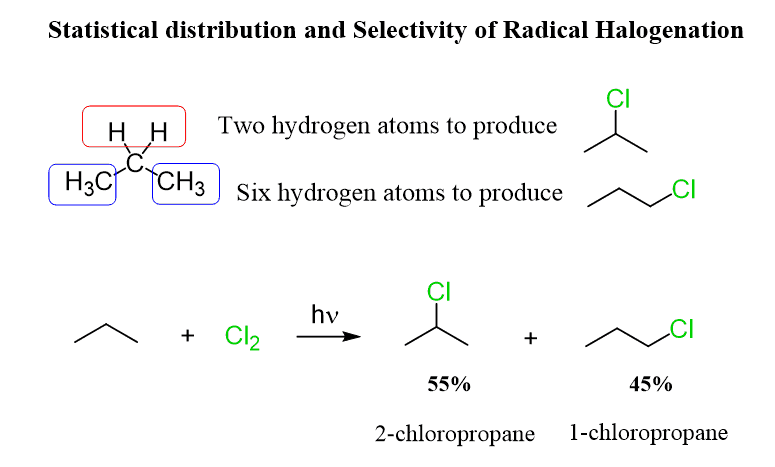
So, the probability ratio is 6:2 = 3:1 in favor of primary hydrogens. However, the experimental observations are reversed for both chlorination and bromination. The approximate 60% to 40% ratio of 2-chloropropane and 1-chloropropane indicates that the selectivity of chlorination for a secondary carbon is 4.5 times higher compared to a primary carbon.
The selectivity of the bromination for secondary carbons is a lot more pronounced and comes to about 82 times.
As expected, the selectivity of radical bromination is even higher for tertiary carbons since tertiary radicals are more stable than secondary radicals:

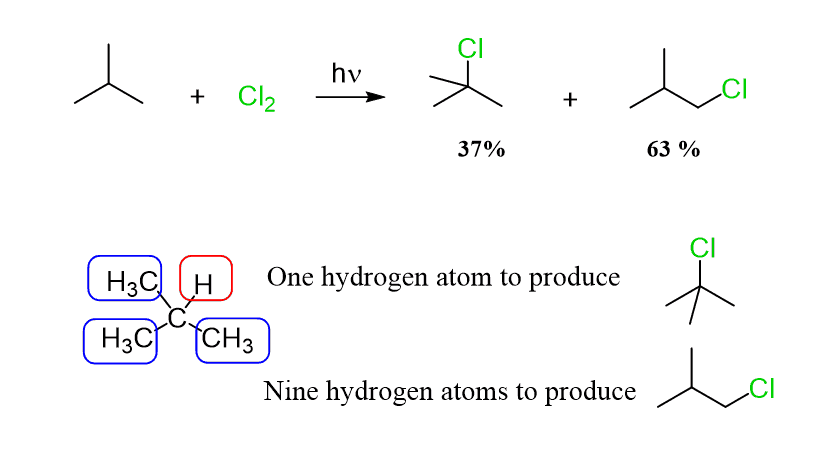
Interestingly, the major product of the chlorination reaction of 2-methylpropane is 1-chloro-2-methylpropane:
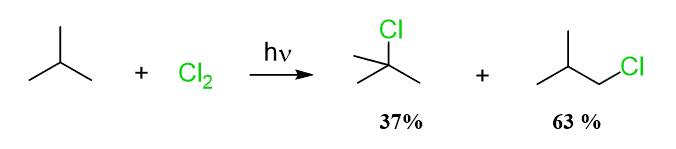
What is going on here?!
Well, everything we talked about regarding the reactivity and selectivity for the halogenation reactions is still true.
However, the ratio of primary hydrogens to the only tertiary hydrogen in a molecule of 2-methyl propane is 9:1, which forms the deceiving image of a reversed reactivity, whereas the tertiary carbon is about 5 times reactive towards the chlorination:
For practical application, we don’t care about the reactivity but rather the percentage of the major product, and that is why bromination is the route to selective halogenation.
On a side note, remember that radical fluorination is a vigorous reaction that you want to avoid, and iodination is too slow to be useful.
Rearrangements in Radical Reactions
Even though radicals are sp2-hybridized and follow the same pattern of stability as carbocations, you need to remember that radicals do not undergo rearrangements!
For example, the following molecule has primary, secondary, and quaternary carbons its bromination is expected to occur on the secondary carbon:
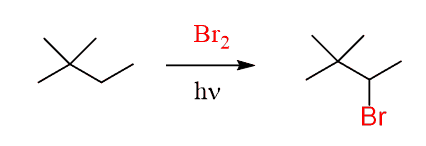
And in fact, it does. The point is, it does not undergo a rearrangement of the secondary carbon to a tertiary carbon by an imaginary methyl radical shift:
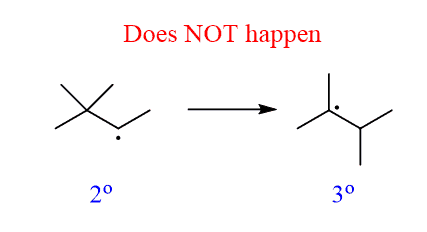
Notice that there cannot be a halogenation of a quaternary carbon since there is no hydrogen on it.
Do not confuse the rearrangement with resonance structures, though.
For example, the product of the halogenation in the following reaction may look like it is a result of some sort of rearrangement, but it is simply the resonance-stabilization of the radical intermediate that leads to its formation:
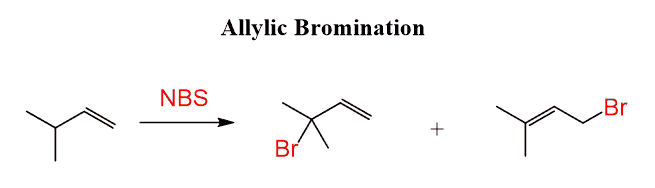
This is a special type of radical reaction called allylic bromination, where the bromine appears on the carbon(s) next to the double bond.
Check Also:
- Free-Radical Addition of HBr: Anti-Markovnikov Addition
- Initiation, Propagation, and Termination in Radical Reactions
- Selectivity in Radical Halogenation
- Stability of Radicals
- Resonance Structures of Radicals
- Stereochemistry of Radical Halogenation
- Allylic Bromination
- Radical Halogenation in Organic Synthesis