Naming alcohols follows the same rules we discussed earlier for the IUPAC nomenclature rules for alkanes.
This is the brief summary of steps:
Step 1. Identify the parent chain.
Step 2. Identify the substituents.
Step 3. Number the parent chain giving the OH group the lowest locant
Step 4. Put everything together having the substituents in alphabetical order.
There are, of course, some deviations since we now have a new functional group – the hydroxyl group.
These are the changes you need to take into consideration.
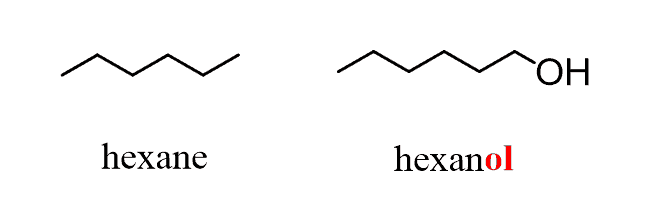
1) The presence of a hydroxyl is identified by changing the parent suffix from “e” to “ol”:
2) Choose the parent chain such that it is the longest carbon chain containing the carbon atom connected to the OH group:

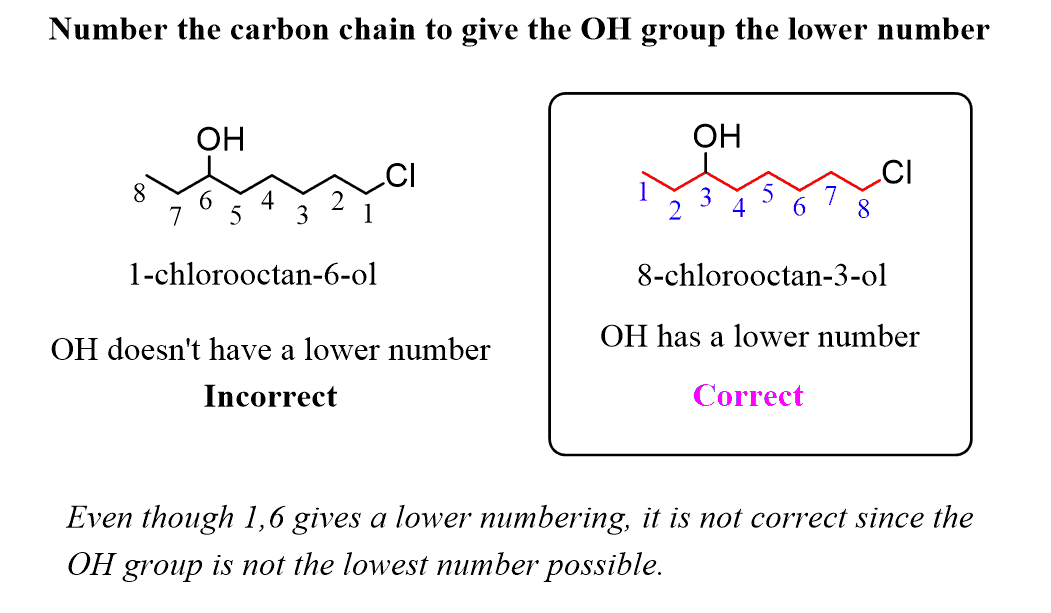
3) The hydroxyl group has a higher priority than alkyl substituents or π bonds. Therefore, you need to number the parent chain such that the OH gets the lowest number possible:
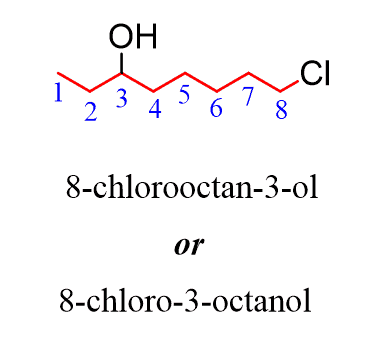
The locant indicating the position of the hydroxyl group can be placed before the parent or before the suffix “ol.” Both names are acceptable according to the IUPAC recommendations:
If the OH group is connected to a chiral center, you will also need to include the absolute configuration at the beginning of the name. Also, if there is a double, the E and Z configuration should be addressed when applicable:
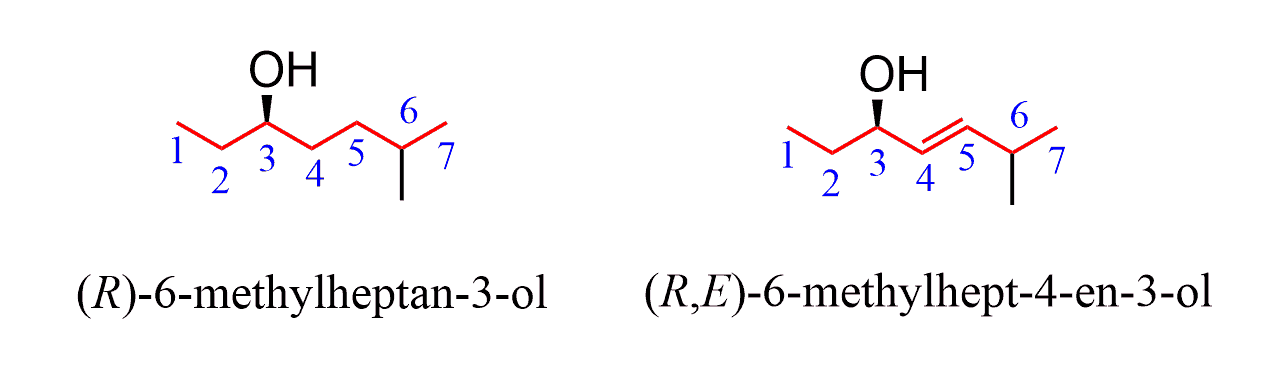
When naming a cyclic alcohol, start numbering the ring beginning with the carbon connected to the OH group. This rule always puts the OH group at C1, therefore, the “1” is usually omitted from the name:
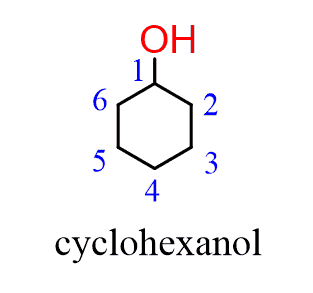
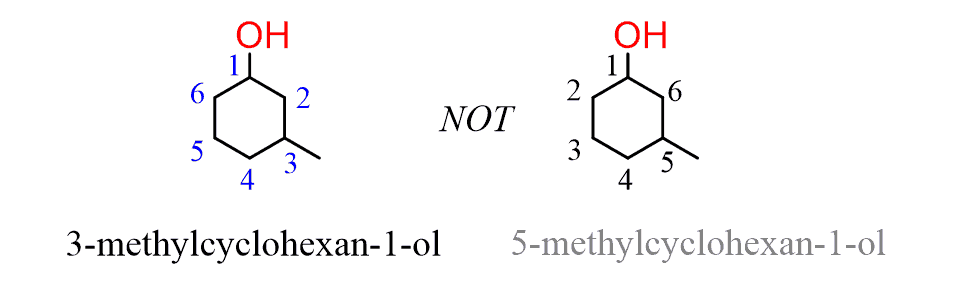
When other groups are present on the ring, it is numbered clockwise or counterclockwise depending on which direction gives the next substituent the lower number:
Alcohols can also be identified by their common names:
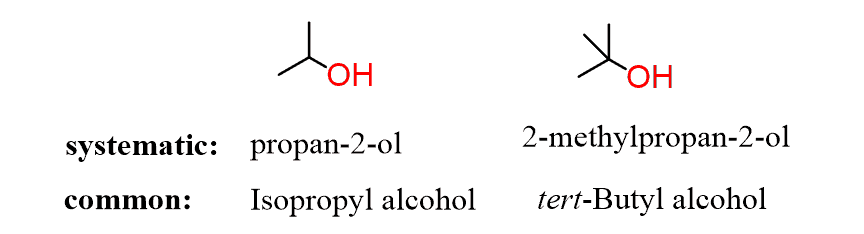


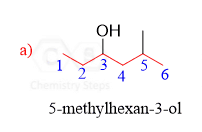
Well explained thanks
This q. Good for our practice.
I think your answer for problem … is wrong.
on carbon 2 there is a 1-methylpropyl group
should the correct answer be…
4-ethyl-7,8-dimethyl-2-(1-methylpropyl)-1-nonanol
1-methylpropyl is the systematic name of sec-butyl, and yes, according to the new IUPAC rules, 1-methylpropyl is the correct way of naming it. Sec-butyl is still widely acceptable in most textbooks thus by many professors. I have a paragraph “IUPAC Moments” about this in the nomenclature post which you can find here.