We have already talked about the ortho, para and meta directions in electrophilic aromatic substitution and their combined effect for disubstituted benzene.
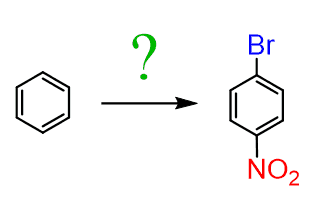
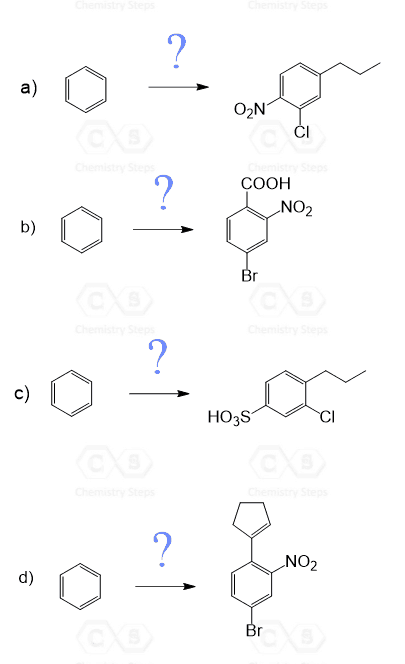
This should allow us to synthesize different aromatic compounds starting from benzene. For example, how can we synthesize 4-bromonitrobenzene from benzene?
There are two groups on the benzene ring and to synthesize any derivative of it, you need to add the groups in the correct order!

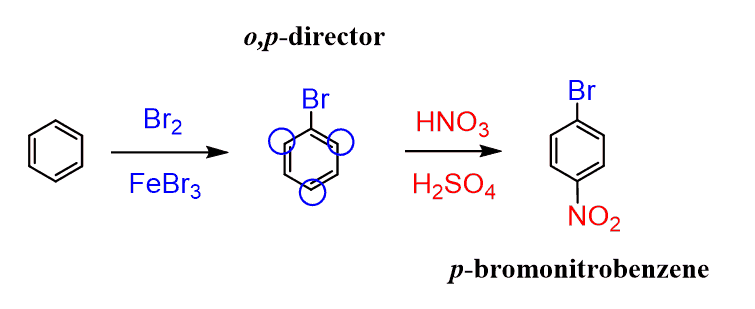
In 4-bromonitrobenzene, the substituents are at para positions of each other (it is also called para-bromonitrobenzene). This means whichever group was added first should be a para director. Recall that halogens are ortho, para directors even though they are deactivators. Therefore, we would first do a bromination of the benzene ring and react the bromobenzene with a mixture of concentrated nitric and sulfuric acids:
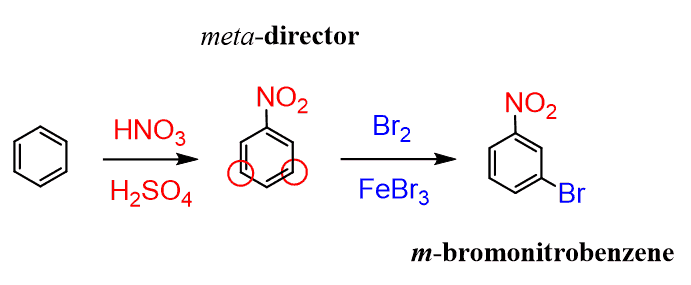
Notice that if we were to add the nitro group first, in the next reaction the Br would have been added to its meta position as the nitro group is strong deactivator and orients the electrophile to the meat position:
Use this table to practice the following exercises. Each compound is synthesized from benzene:


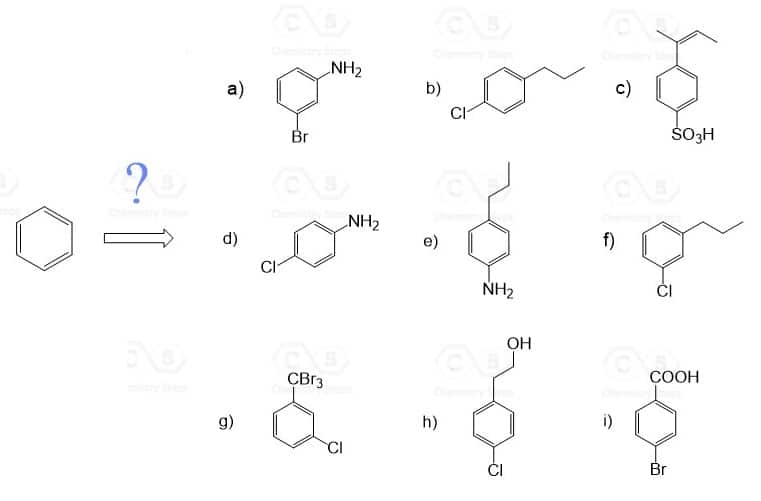

Hi! 2b doesn’t have an answer displayed when you click on the plus.
Hi there, thanks for letting me know – added.