We have seen many different addition reactions of alkenes and conjugated systems. One of them was the reaction of alkenes with bromine. Bromine is a red liquid and it is an indicator for the presence of alkenes since the double bond reacts very quickly decolorizing its red solution:
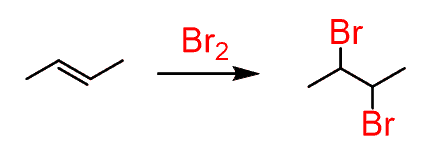
The reaction works with conjugated dienes and trienes as well:
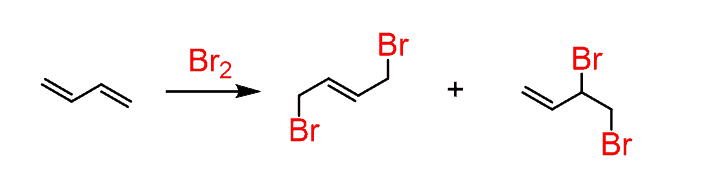
It even works with cyclic dienes:
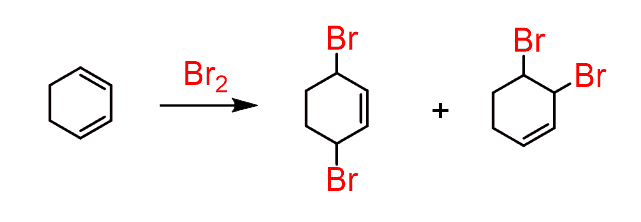
Now, why do we say, “even with cyclic dienes”?
That’s because when we add another double bond to the cyclohexadiene, we get benzene and interestingly, it does not react with Br2 anymore:

No electrophilic addition to the double bonds of benzene occurs – at least not under the conditions when alkenes react readily.
The chemical properties of benzene and aromatic compounds is a separate topic, and we will get to this many times. However, the take-home lesson from the comparison of reactions above is the unusual stability of benzene and aromatic compounds in general.
Benzene is the first aromatic compound that was studied, and its structure is in the basis of aromaticity. Even though not all the aromatic compounds are derivatives of benzene, for starters, take benzene as the symbol of aromatic compounds.
How was Benzene Discovered
This has an interesting historic background.
Benzene was isolated in 1825 by Michael Faraday from the oily residue in the illuminating gas lines in London. And ever since the discovery of its unusual stability towards electrophilic addition reactions, chemists tried to come up with a structure that would explain this feature.
The most successful was August Kekulé who, in 1865, proposed a structure which essentially is accurate except for a detail that we’ll discuss in a moment.
Kekulé suggested that benzene is an equilibrium mixture of two compounds with alternating double bonds:

These structures are called Kekulé structures.
You have probably noticed the inaccuracy of the Kekulé’s description;
These two structures are not different compounds – they are two resonance structures of benzene since all the atoms are connected the same way and only the electron distribution is different:

Another interesting fact about benzene and other aromatic compounds is their characteristic odor for which they were named aromatic. However, it is not the odor that unifies and makes them special but rather their chemical properties – specifically their unusual stability.
So, where is the Stability of Benzene coming from? Does it have to do with the cyclic structure?
Well, not really because we saw that cyclohexadiene reacts with Br2:

Does it have to do with a cyclic compound being completely conjugated?
Again, not necessarily. For example, cyclooctatetraene (abbreviated as COT) is a cyclic, conjugated (that’s how it looks like, however it is not – we’ll see in the net post) compound, but it reacts readily with bromine even at negative temperatures:

Albright then – what is the structure of benzene that makes so stable? This was a question for years and numerous possibilities were suggested after Kekulé.
In 1869, Albert Ladenburg proposed a structure for benzene called Ladenburg benzene or prismane:
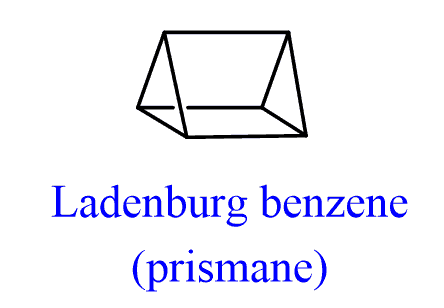
Hopefully, this structure does not look like being very stable to you. Remember, the simple cycloalkanes – cyclopropane is not stable because of a high ring strain and cramming two of these rings together shouldn’t make it any more stable.
The question is, what made the 19th century chemist think that this was a suitable structure for benzene? And the answer is the lack of double bonds which would explain its tolerance to bromine.
This compound was synthesized in 1973 by Professor Thomas J. Katz group at Columbia University. As expected, it was very unstable and turned out to be an explosive liquid.
Kekule structure was eventually agreed to be the best candidate among others since all the efforts to prepare the alkene 1,3,5-cyclohexatriene led to the formation of benzene which was a strong indication that 1,3,5-cyclohexatriene and benzene must be the same compound, which we know they are.
The structure was expected and confirmed but it does not answer what makes benzene so stable and unreactive in electrophilic addition reactions.
The next post will be dedicated to answering this question by exploring the structure of benzene.
Before moving on, let’s also mention a few important classes of aromatic compounds and their application.
Importance of Aromatic Compounds
The unusual stability makes aromatic compounds very common in natural systems such as for example the DNA which contains nucleobases responsible for the genetic information. Adenine, Guanine, Thymine and Cytosine are all aromatic compounds:

What are the key patterns to recognize here when comparing to benzene? First, remember that it is cyclic (all the aromatic compounds are cyclic), and it has a double bond on every second carbon. It goes interchangeably single-double. Here are also some important molecules that are not natural and prepared through organic synthesis:
Check this 60-question, Multiple-Choice Quiz with a 1.5-hour Video Solution covering the naming and electrophilic aromatic substitution reactions.
Aromatic Compounds
Check Also:
- Naming Aromatic Compounds
- Benzene – Aromatic Structure and Stability
Aromaticity and Huckel’s Rule - Identify Aromatic, Antiaromatic, or Nonaromatic Compounds
- Frost Circle
- Annulenes
- Electrophilic Aromatic Substitution – The Mechanism
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- The Alkylation of Benzene by Acylation-Reduction
- Ortho Para Meta Directors in Electrophilic Aromatic Substitution with Practice Problems
- Ortho Para and Meta in Disubstituted Benzenes
- Why Are Halogens Ortho-, Para- Directors yet Deactivators?
- Limitations on Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Electrophilic Aromatic Substitution with Arenediazonium Salts
- Reactions at the Benzylic Position


This is nice. I am not sure I understand the part about DNA bases though. None of them look aromatic except adenine…?
That is a good observation. You will need to draw resonance structures of the nucleobases to see their aromatic structure.
Thanks so much for this help
You are welcome.