Ozonolysis of Alkynes
The Ozonolysis is an Oxidative Cleavage reaction where both the π and σ bonds of the double or triple bond are broken to form two carbonyl groups. If you remember, in the case of alkenes, either ketones or aldehydes were formed depending on whether it was an internal or an external double bond:
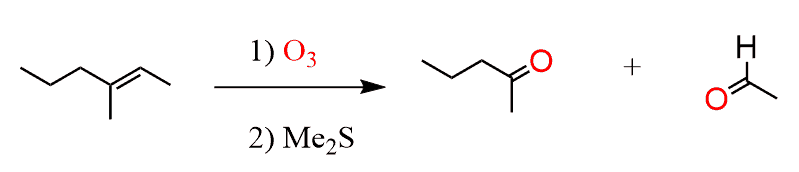
Check the ozonolysis of alkenes for the mechanism.
Just like this, alkynes also give different products when the triple bond is external or internal. However, this time it is neither a ketone nor an aldehyde.
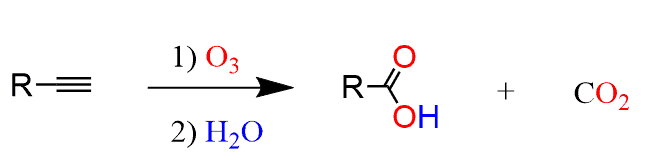
For external alkynes, the ozonolysis results in a carboxylic acid and carbon dioxide:
The ozonolysis of internal alkynes, on the other hand, produces two carboxylic acids:
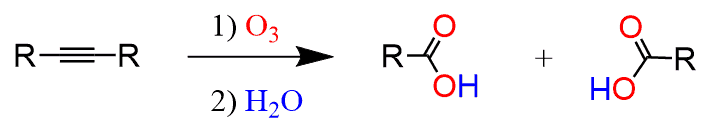
So, here if the alkyne is symmetrical, meaning the R groups are identical, then two molecules of the same carboxylic acid are formed. If the alkyne is not symmetrical, then two different carboxylic acids are formed:
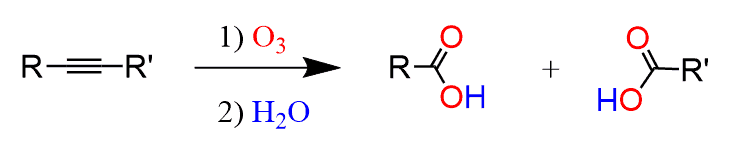
How to determine the product of the ozonolysis reaction
If you need to determine the product(s) of an ozonolysis reaction, number the main hydrocarbon chain of the alkyne:
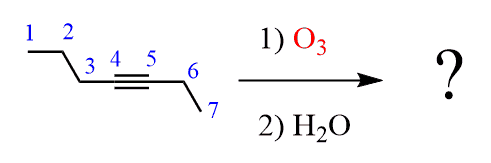
In the product, erase the triple bond and replace it with a carboxylic acid (for an internal alkyne) or a carbon dioxide for the external carbon of the triple bond:

Oxidative cleavage with KMnO4
The oxidative cleavage of alkynes can also be achieved by using potassium permanganate (KMnO4):
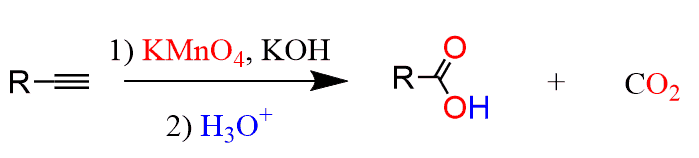
And, just like in the ozonolysis of internal alkynes, two carboxylic acids are produced:
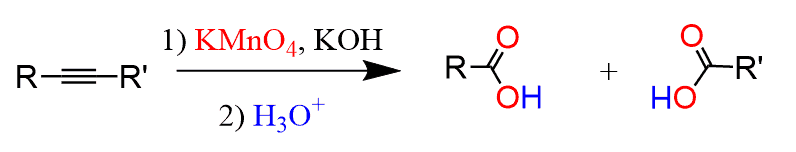
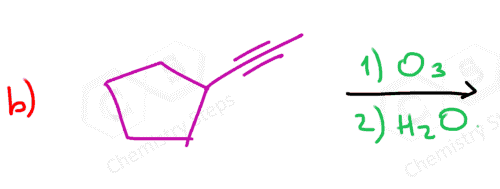
In the following Practice Problems, we will determine the products for ozonolysis of alkynes and alkenes as well as retrosynthetic analysis to identify the starting alkyne and alkene in the ozonolysis reactions.




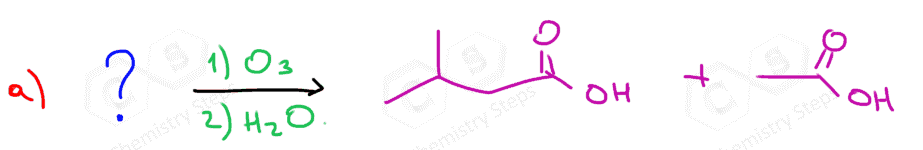

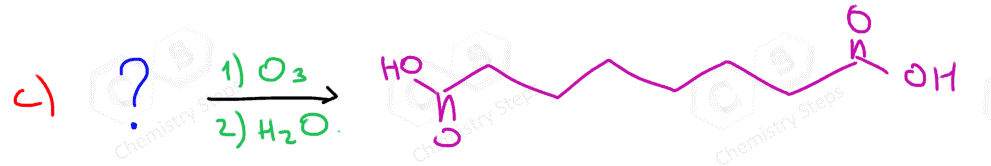
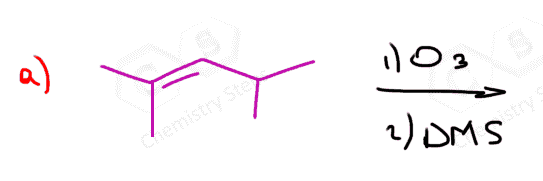
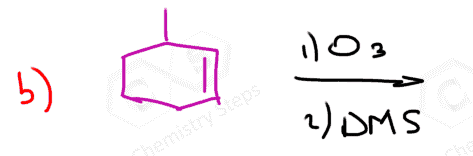
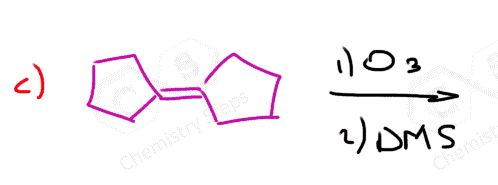
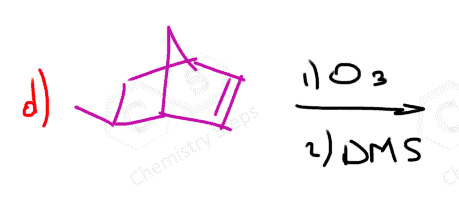
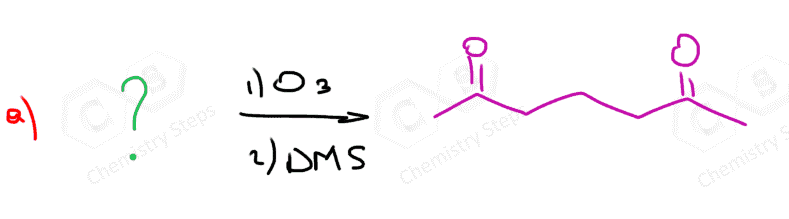
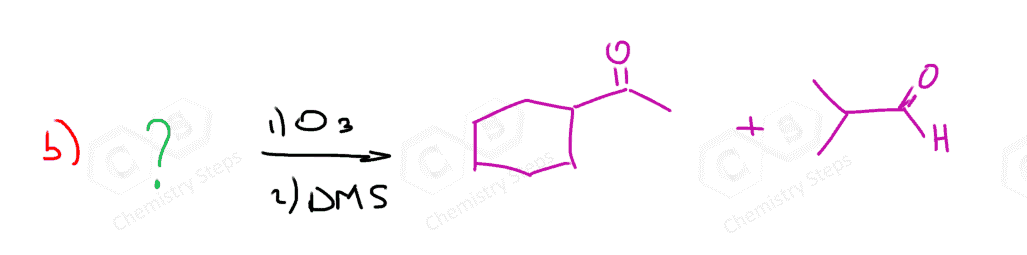
thank you so much i had such a hard time finding practice problems for this concept!
You are welcome. I am glad it helped you.