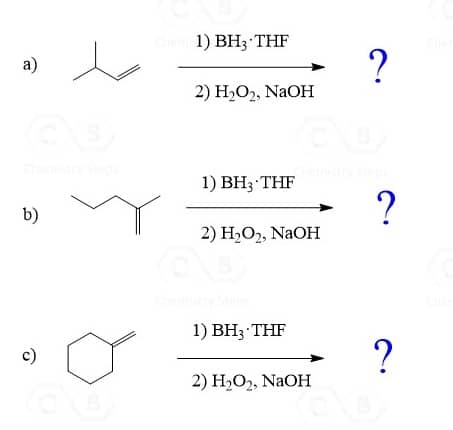
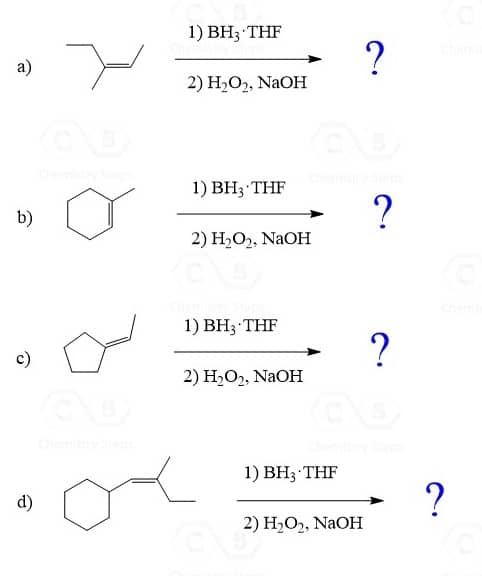
The Regiochemistry of Hydroboration-Oxidation
In the previous post, we mentioned that Hydroboration-oxidation places the OH group on the less substituted carbon of an alkene:

There are two reasons for this: steric and electronic. This is a result of a preferred alignment of the BH3 and the alkene:
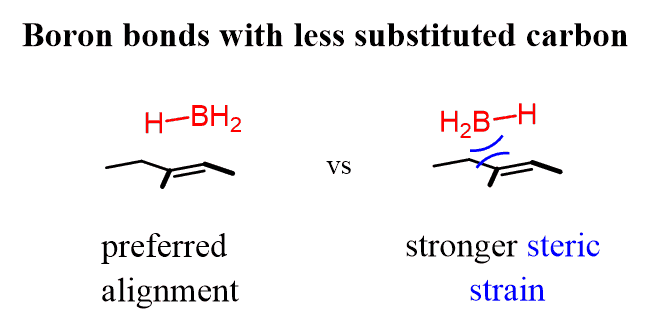
The first alignment minimizes the steric strain between the boron and the carbon since it is next to a secondary carbon, while the second possibility puts it next to a tertiary carbon.
The other reason for this regioselectivity is the stability of the transition state connecting the boron and the less substituted carbon. Because the alkene is the electron donor and the boron is the electron acceptor, the first alignment puts a partial negative charge on the boron and a partial positive charge on the more substituted carbon in the transition state:

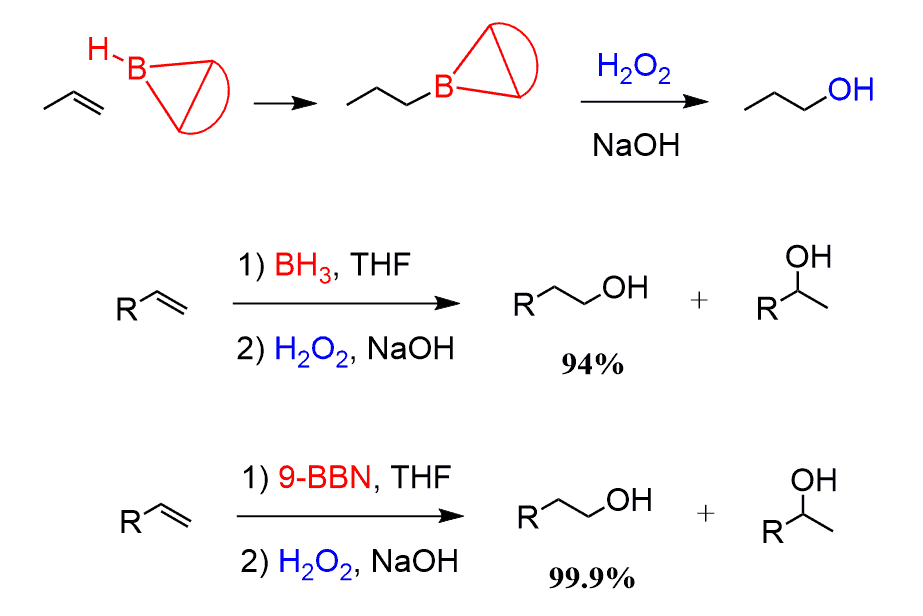
To increase the steric factor and improve the regioselectivity of the hydroboration, dialkyl boranes are used instead of BH3. The most common one is 9-Borabicyclo[3.3.1]nonane or 9-BBN:

As an example, it increases the regioselectivity of the Hydration-oxidation of Ethylene Derivatives to almost 100 : 0.
The Stereochemistry of Hydroboration-Oxidation
First, remember that Hydroboration is a concerted process, and the BH2 and H species add to the double bond simultaneously. As a result, they both appear on the same side of the alkyl borane. This is called a syn addition:
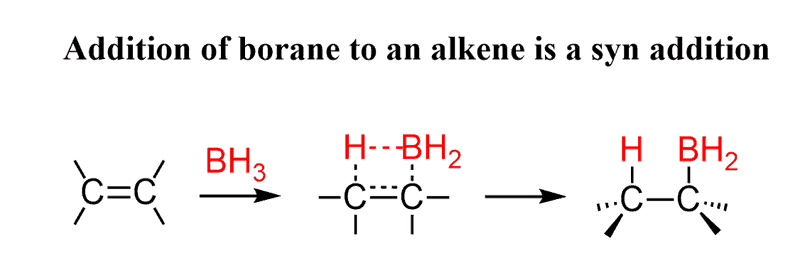
This addition can and does occur from both faces of the double bond:
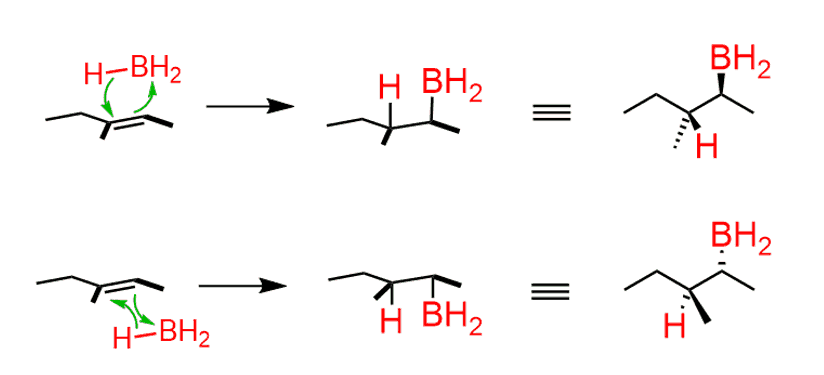
Notice that in both cases, the H and BH2 appear on the same side (syn), but the alkyl boranes are enantiomers.
During the oxidation of the alkyl boranes in the following step, the absolute configuration of both stereogenic centers is retained:

This leads to the formation of a pair of enantiomeric alcohols: