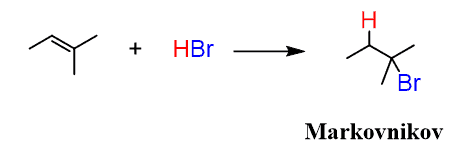
We have seen that the electrophilic addition of hydrogen halides to alkenes produces alkyl halides according to Markovnikov’s rule:
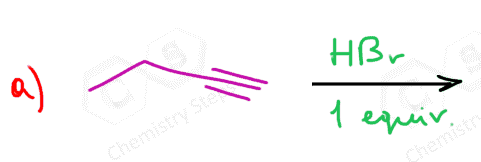
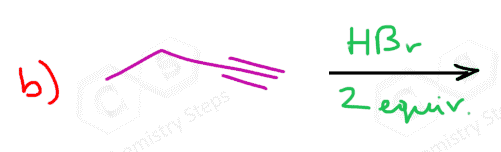
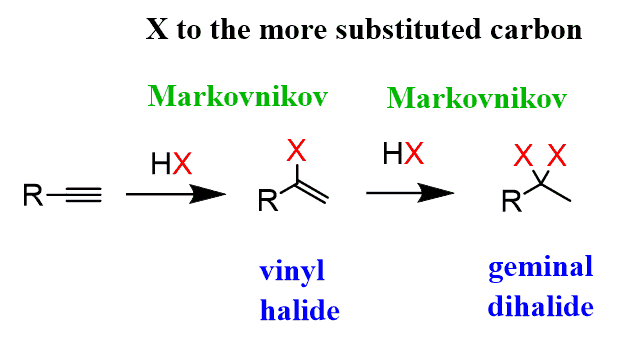
Alkynes undergo hydrohalogenation following a similar pattern. The addition of one equivalent of HX produces a vinyl halide, which is an alkene that is converted into a geminal dihalide upon the second electrophilic addition of HX:

To distinguish geminal and vicinal, you can go with geminal as being “jammed” on one carbon:

More scientifically, it comes from the Latin geminus – “twin.”
The Mechanism and Regiochemistry of Alkyne Hydrohalogenation
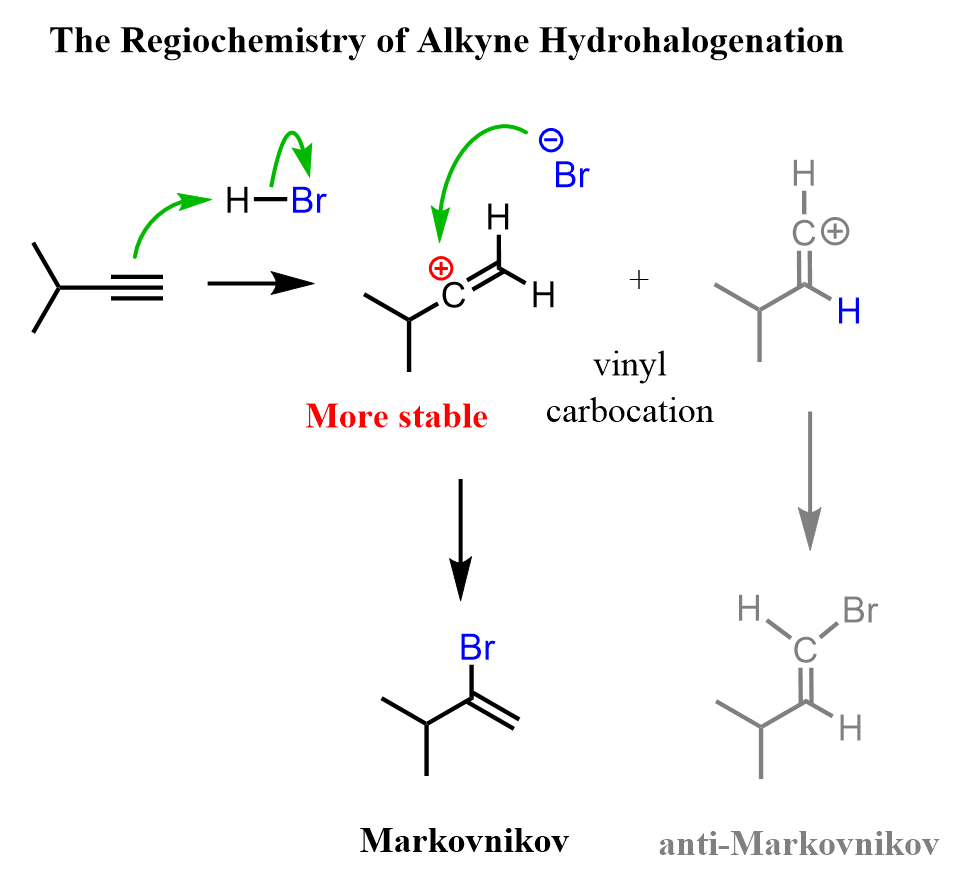
Like in alkenes, it starts with the addition of H+ to the π bond, forming an sp-hybridized vinyl cation:

The more stable 2o carbocation is then attacked by the Cl-, forming a vinyl chloride:
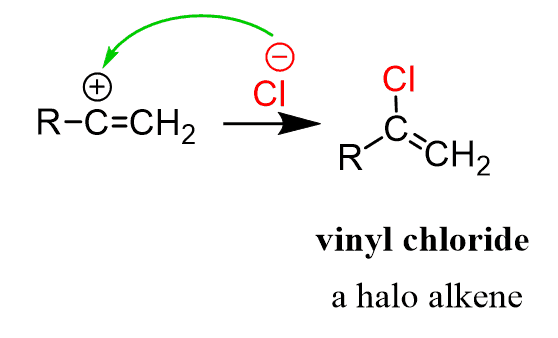
The resulting haloalkene is less reactive than the unsubstituted alkene since the electronegative halogen decreases the electron density of the double bond by the inductive effect. Therefore, the reaction can usually be stopped at this step if needed. Let’s summarize the mechanism of the hydrohalogenation of alkynes:
The second addition of the acid starts with the protonation of the vinyl halide:

Two carbocations are possible here, and each has an argument to be supported. On one hand, carbocation A is a secondary carbocation and, from that perspective, should be more stable than carbocation B. On the other hand, the positive charge on A is next to the more electronegative Cl atom, and this is not a good combination.
However, there is also the factor of resonance-stabilization, which is only possible for the carbocation A:

And this is the determining factor that favors the formation of carbocation A. Later on, we will also see how the resonance stabilization determines the directing effect of halogens in electrophilic aromatic substitution.
Notice also that in the second resonance structure, all the atoms have a complete octet, which is one of the key factors for determining the resonance stability and major resonance contributor.
After the second nucleophilic attack of the Cl–, a geminal dihalide is formed:
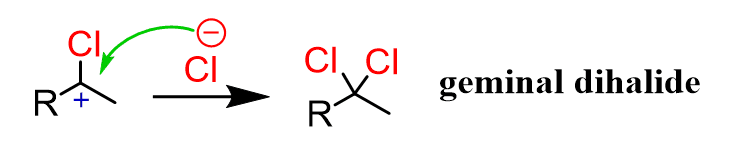
Looking at the overall addition of HX to alkynes, we can see that it is a regioselective reaction following Markovnikov’s rule since, first, the more substituted carbocation is formed, and for the vinyl halide, a resonance-stabilized carbocation is formed.
The Stereochemistry of Alkyne Hydrohalogenation
If an internal alkyne is used, there is no regiochemistry involved, and a mixture of E and Z isomers is obtained:
Just like for alkenes, anti-Markovnikov addition of hydrogen bromide to alkynes can be achieved when the reaction is carried out in the presence of peroxides.
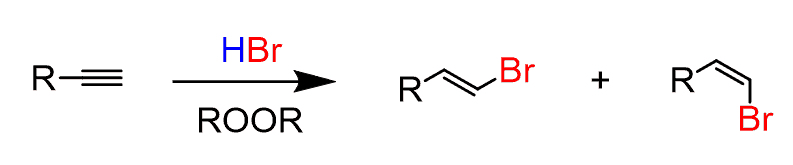
Only HBr works for anti-Markovnikov addition to terminal alkynes. E and Z alkenes are formed.
The reaction goes by a free-radical mechanism.