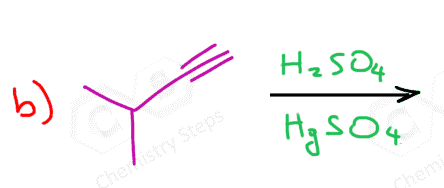We have seen that in acid-catalyzed hydration of alkenes, an alcohol is formed according to the Markovnikov’s rule:

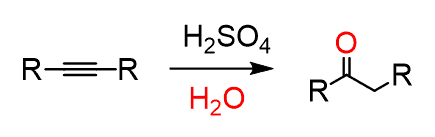
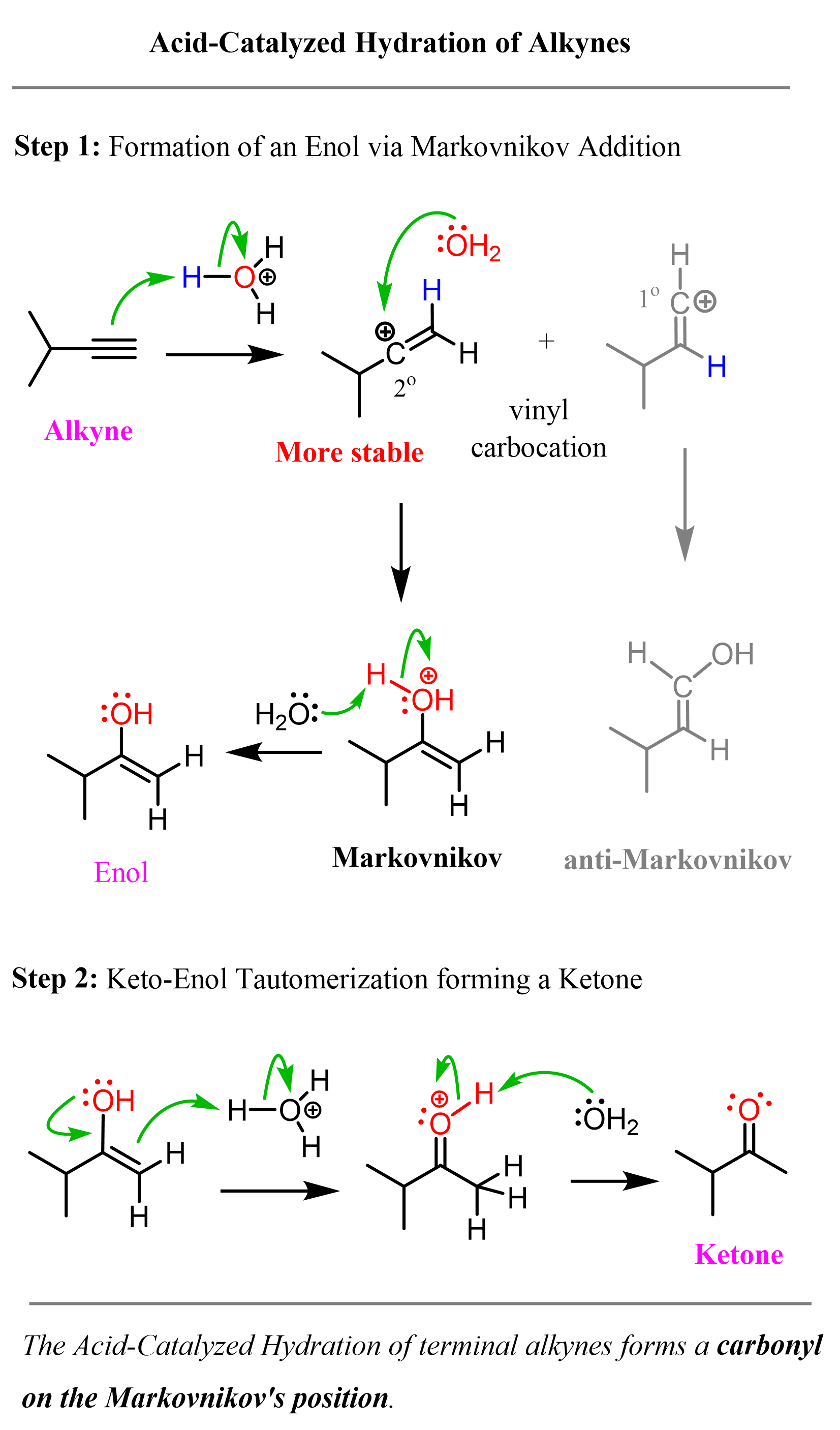
In a similar way, alkynes also undergo acid-catalyzed hydration. The final product of this reaction, however, is a ketone:
Let’s start with internal alkynes. The first step is the protonation of the triple bond forming a vinyl carbocation:
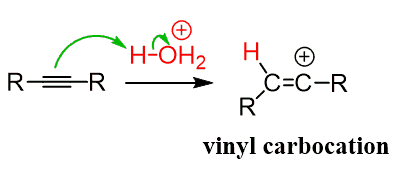
This is followed by a nucleophilic attack of the vinyl carbocation by the water:

In this step, an alcohol, where the OH group is connected to a C=C double bond is formed. This alcohol is called an enol; ene (alkene) and ol (alcohol).
Enols are generally unstable and rearrange into ketones (internal) or aldehydes (terminal OH) as soon as they are formed:
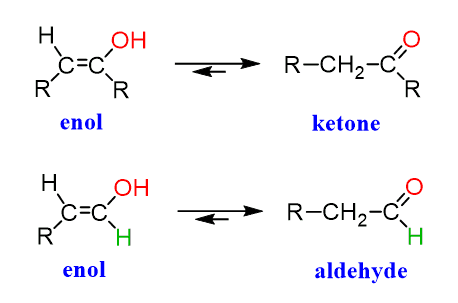
These molecules are constitutional isomers, and their interconversion is called keto-enol tautomerization. In acidic solution, we can show the mechanism of the tautomerization as follows:

Let’s summarize the mechanism of acid-catalyzed hydration of alkynes. In the first, rate-determining step, the triple bond is protonated, forming the more substituted vinyl carbocation which is attacked by water, forming an intermediate called an enol. The enol is then transformed into a ketone via keto-enol tautomerization:
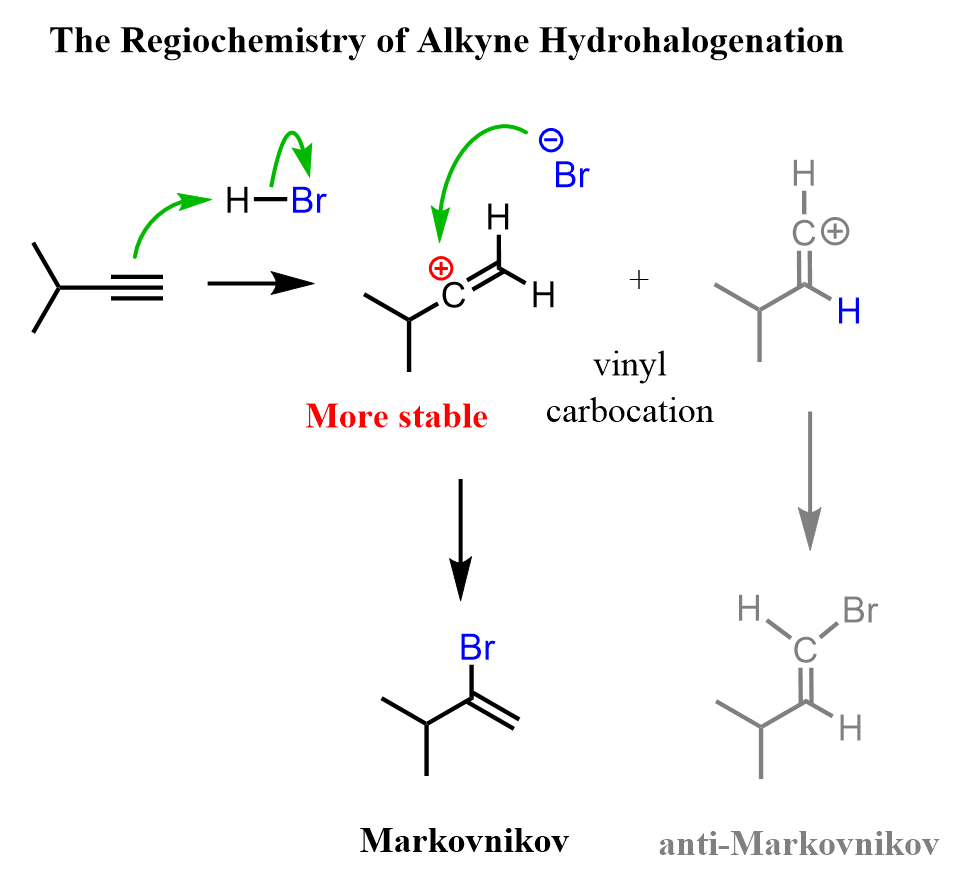
Regiochemistry
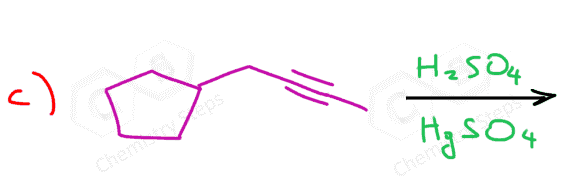
The acid-catalyzed hydration of terminal alkynes produces a ketone because the enol formation is a Markovnikov addition. The reason for this is that vinyl carbocations have the same stability pattern as regular carbocations: the more substituted, the more stable. As an example, we can see the mechanism of hydrobromination of a terminal alkyne:
As a shortcut to acid-catalyzed hydration of terminal alkynes, remember that it forms a carbonyl in the Markovnikov’s position, thus the final product is a ketone:

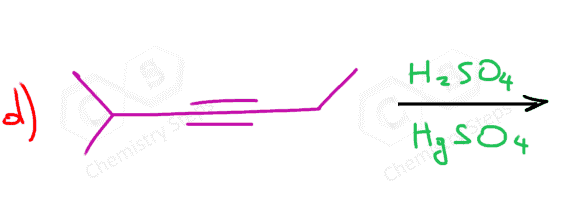
The acid-catalyzed hydration, or any other hydration reaction of symmetrical alkynes, produces one ketone:
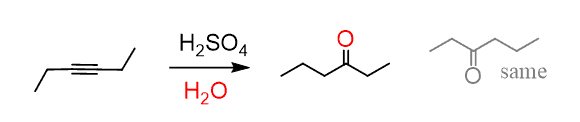
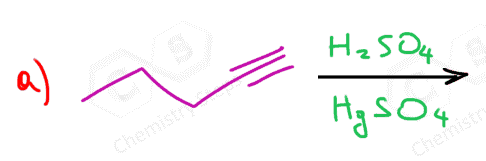
Unsymmetrical internal alkynes show no preference as to which carbon gets the O,H and as a result, a mixture of two ketones is obtained:

Because of the slower addition reactions of alkynes compared to alkenes, HgSO4 is sometimes used as a catalyst:
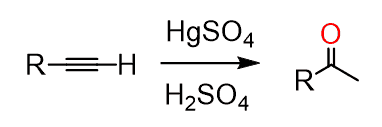
The d electrons and empty d orbital of the mercury allow for the formation of a cyclic intermediate, much like we have seen in the formation of the halonium ion and oxymercuration of alkenes. The nucleophilic attack of this cyclic intermediate by water occurs at the more substituted carbon atom since this leads to a more stable transition state:

Therefore, the hydration of a terminal alkyne produces a ketone through a keto-enol tautomerization of the more substituted enol.
To summarize, we learned that acid-catalyzed hydration and oxymercuration of alkynes convert them to ketones. Both reactions are Markovnikov additions; therefore, terminal alkynes give one ketone. Unsymmetrical internal alkynes give a mixture of two ketones, while symmetrical internal alkynes can only give one ketone.
Terminal alkynes can also be hydrated in an anti-Markovnikov fashion, forming aldehydes by Hydroboration-Oxidation, and we will discuss this in the next article: