We learned earlier that according to Markovnikov’s rule, the addition of an HX acid to an alkene produces the more substituted alkyl halide as the major product:
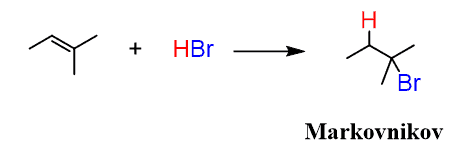
Interestingly, in the presence of peroxides, HBr forms the less substituted alkyl bromide:
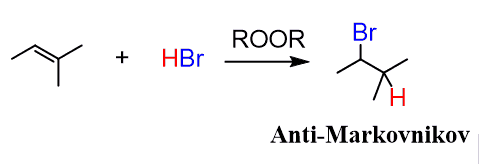
This is called anti-Markovnikov, free radical addition to an alkene, and is only efficient when the HBr is used. Let’s see how this happens.
The Mechanism of Radical Addition
The Markovnikov addition of HX acids goes by an ionic mechanism, and the regioselectivity is dictated by the stability of the more substituted carbocation:

The reaction goes by a radical mechanism when a peroxide is present. The first step is the homolysis (homolytic cleavage) of the RO-OR bond:
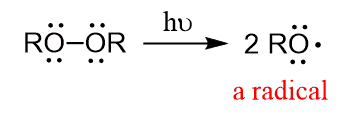
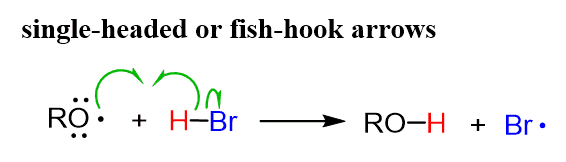
The resulting radical reacts with HBr by abstracting the H, and a new radical Br• is formed:
After this, a regioselective addition of the Br to the alkene happens. It is regioselective because the more substituted one forms as the major intermediate:

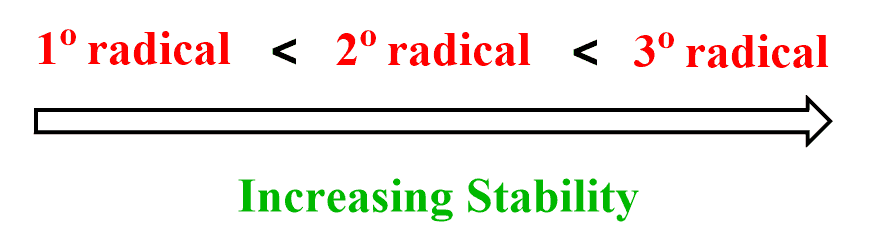
Remember that, just like for the carbocations, radical stability increases with the degree of substitution:
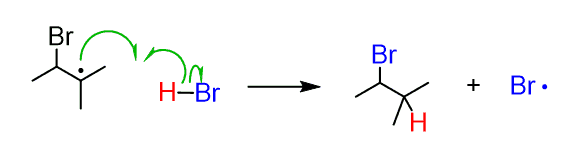
Protonation of the 3o radical by another H abstraction from HBr completes the reaction:
Let’s compare the intermediates in the ionic (Markovnikov) and radical (anti-Markovnikov) reactions to better understand the opposite regioselectivity of the HBr addition:

The key difference is that in the ionic mechanism, the H+ adds first, forming the more stable carbocation, while in the radical addition, Br• adds first to form the more stable radical.
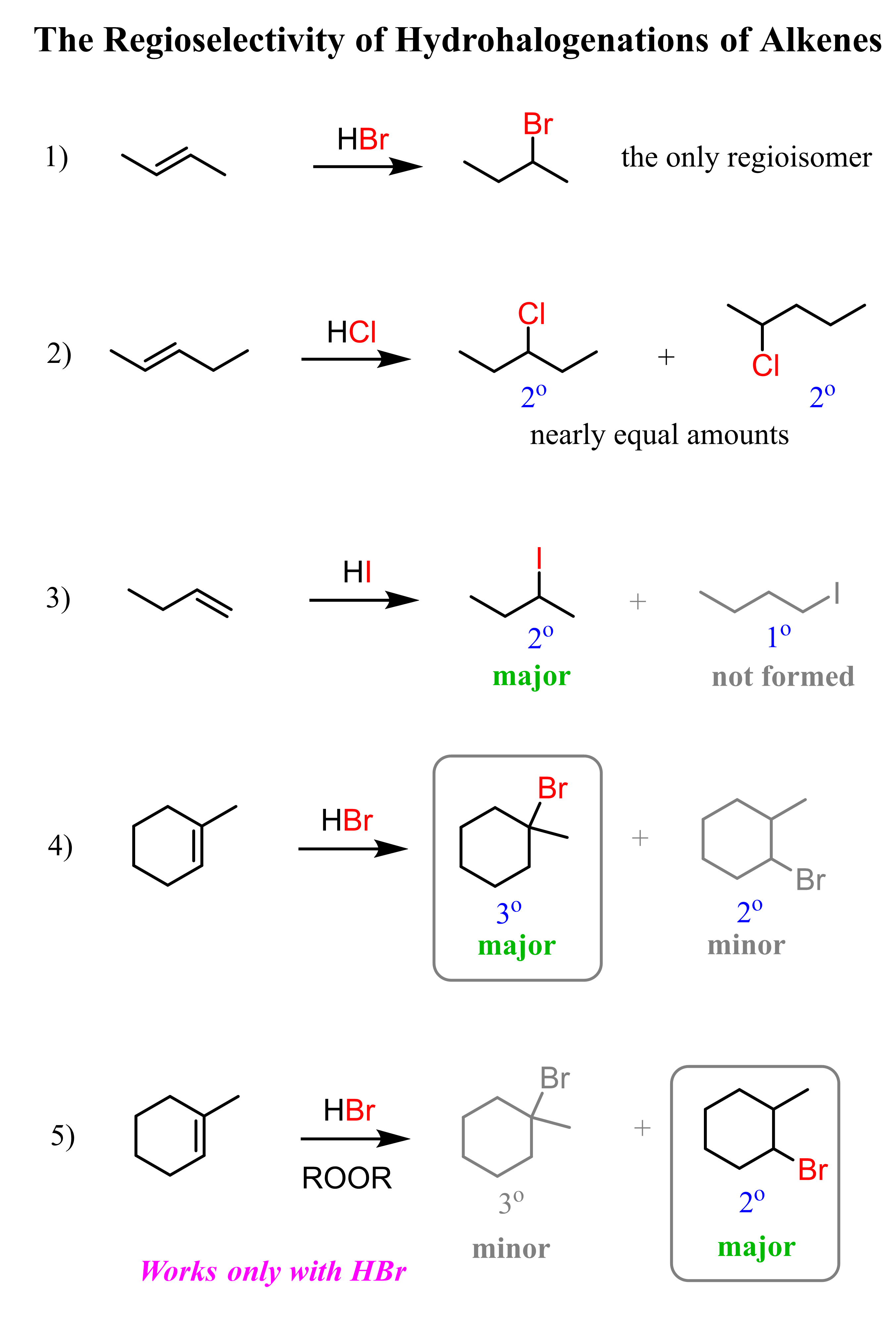
Some examples of regiochemistry in the hydrohalogenation of alkenes for achieving Markovnikov and anti-Markovnikov addition:
Stereochemistry of the HBr Radical Addition
There is no stereoselectivity in radical addition reactions. Syn and anti-addition of the H and Br produce the maximum number of stereoisomers.
Let’s look at the stereochemistry of our reaction and then at another one where the two carbons of the double bond become stereogenic centers.
The Br addition to the alkene happens from both faces of the alkene, producing a new stereogenic center (if possible) in equal amounts:
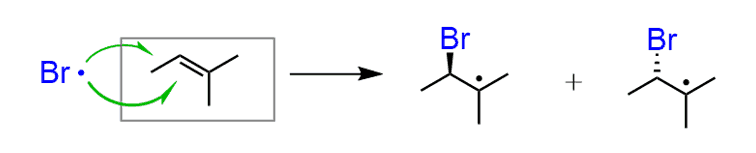
The other carbon of the double bond is now a radical. And radicals are sp2-hybridized; therefore, just like the carbocations, they are planar and react from both faces:
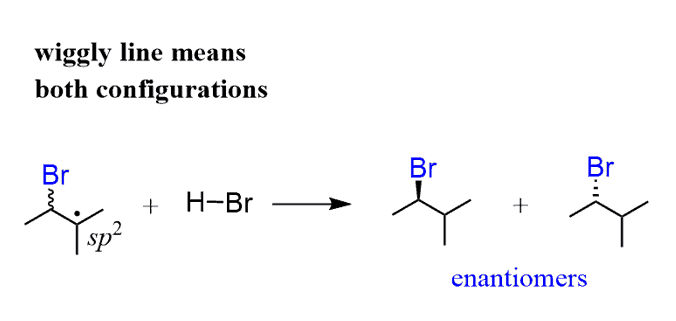
In summary, this reaction produces a racemic mixture of enantiomers following anti-Markovnikov regioselectivity:

If both carbons of the double bond turn into a stereogenic center, then a mixture of enantiomers and diastereomers will be obtained:



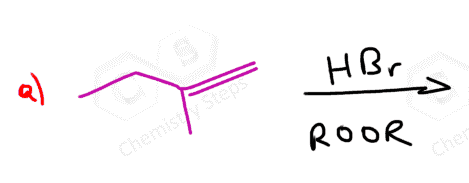
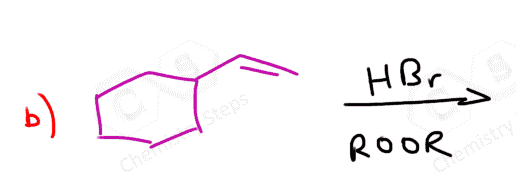
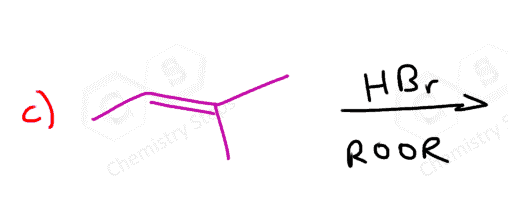
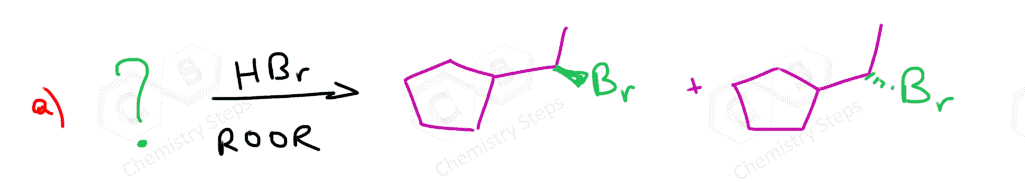
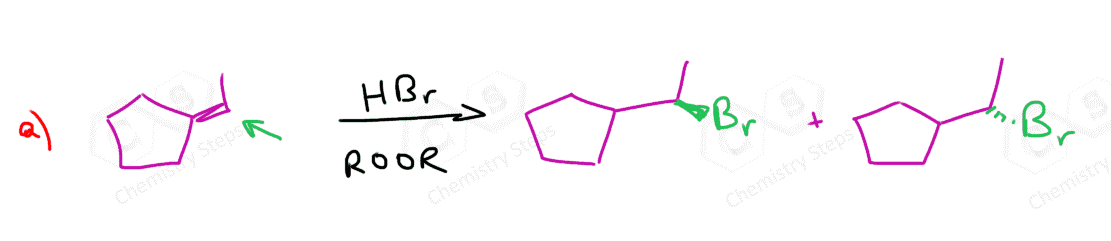

What’s the answer worked out for question #5?
I have added the mechanism and the major product for this reaction.
”If both carbons f the double turn into a stereogenic center, then a mixture of enantiomers and diastereomers will be obtained:” can a radical carbon be stereogenic? doesn’t it have to have 4 different subs/attachments to it?
That’s a good question. Radicals are not chiral centers as the carbon is sp2-hybridized with trigonal planar geometry. This may be different when the carbon is locked in to have a tetrahedral geometry despite having only three atoms connected by single bonds.
As for the diastereomeric products, the term “both carbons turning into stereogenic centers” refers to the final product, and not the radical intermediates.