In this post, we will talk about the stereochemistry of radical halogenation leading to racemic mixture of enantiomers or diastereomers.
Before getting into more details, let’s recall that radicals are similar to carbocations as both are trigonal planar, sp2-hybrydized atoms following the same stability trend:

This pattern of stability determines the selectivity of radical halogenation:
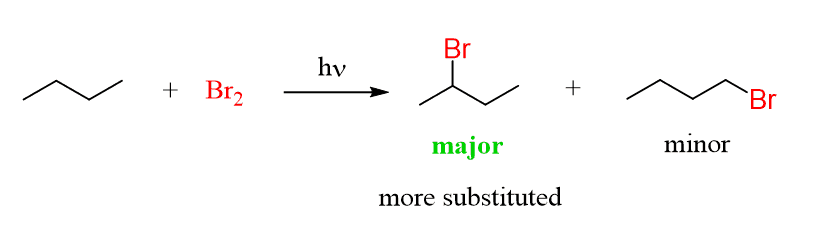
The more substituted carbons are more reactive in radical halogenation and this especially applicable to the bromination where the more substituted haloalkane predominates.
Notice that the primary position is also reactive and some 1-bromobutane (achiral) is also formed.
Now, let’s discuss the stereochemistry of this reaction:
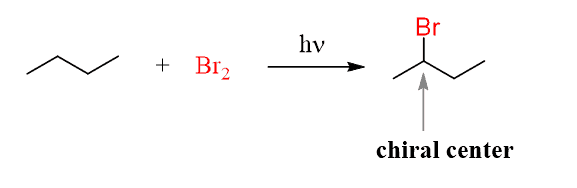
The carbon connected to the bromine turned into a chiral center during the reaction and it is the only chiral center of the molecule.
Therebefore, the molecule can exist as two enantiomers:
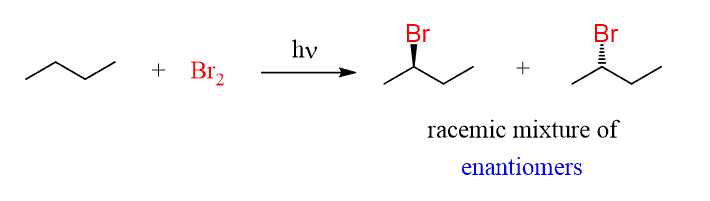
In fact, the major product of this reaction is a racemic mixture of R and S 2-bromobutne.
The formation of a racemic mixture is somewhat similar to the SN1 mechanism, where the carbocation is attacked by the nucleophile from both sides leading to racemization of the newly-forming chiral center:
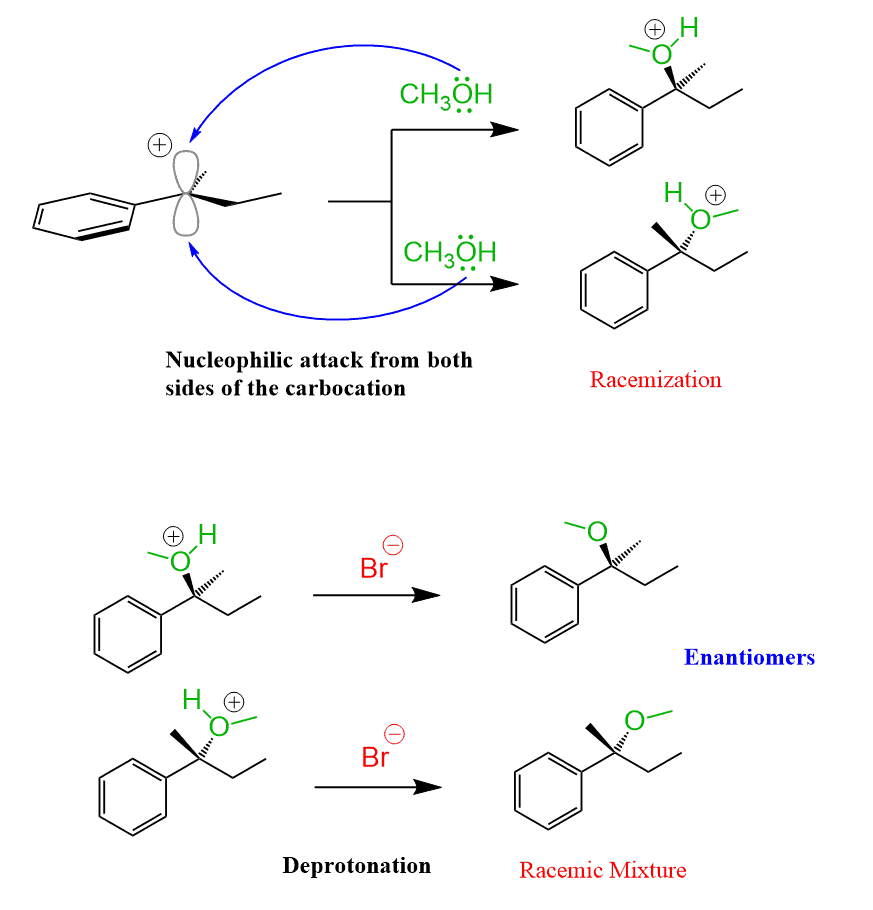
Same here, abstraction of a hydrogen atom from the carbon 2 produces a trigonal planar radical with the unpaired electron in the p orbital. This achiral radical then reacts with bromine at either face just like the carbocations do (remember though – these reactions follow different mechanisms):

The radical is flat and reacts at both faces with equal probability creating a racemic mixture of 2-bromobutane enantiomers.
If the starting alkane contains a chirality center, and this center is where the halogen reacts, then again a racemization occurs:
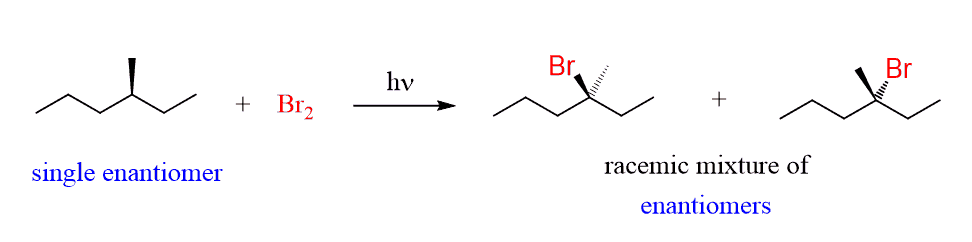
Formation of Diastereomers
Another possibility to consider for the stereochemistry of radical halogenation is then the starting material contains a chirality center that is not involved in the reaction.
If the halogenation produces a new chirality center, then a mixture of diastereomers is obtained:

One thing to mention here is that the presence of a chiral carbon next to the reactivity center influences the stereochemistry of most reactions. And because of this, the diastereomers in the halogenation of chiral substrates may not be formed in equal amounts.

