Monosubstituted Derivatives of Benzene
Benzene is the “first and simplest” aromatic compound and many monosubstituted derivatives of benzene are named systematically by adding the name of the substituent to “benzene” which is the parent: For examples:

If the substituent is an alkyl chain with more carbon atoms than benzene, then benzene can be treated as a substituent. The ring, in this case, is called a phenyl group just like the methyl, ethyl, etc. The phenyl group is often abbreviated as “Ph”.
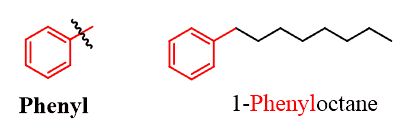
There are also monosubstituted benzene rings that have common names. And when naming an aromatic compound with one of these rings, you need to use the common name as the parent and not the “benzene”. Below is the list of these common names:

Unfortunately, there are no other options and you need to memorize these names. At least the first row since they are more common, and you will encounter them all when dealing with aromatic compounds.
Disubstituted Derivatives of Benzene
Some disubstituted benzene rings also have common names, and the first thing here is to know the relative positions of ortho, meta, and para:

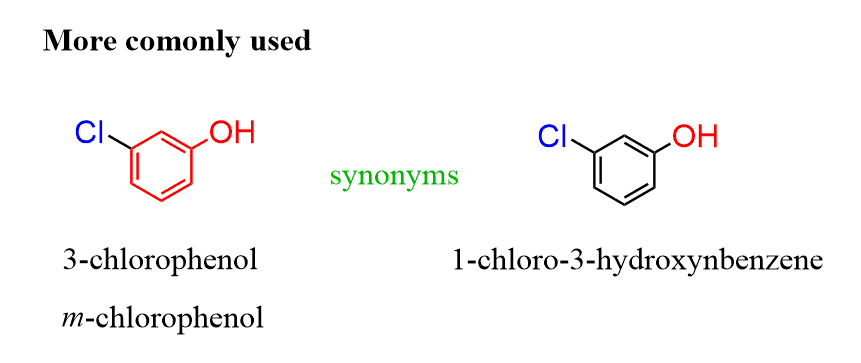
For the other rings with common names, start numbering the ring from the substituent that is part of the common ring such that the other groups get the smallest possible locants:

Using “benzene” as the parent can also be encountered, even though it is not what you’d commonly see:
In general, to name an aromatic compound, you can follow these steps:
- Identify and name the parent. If it is not one of the common names, then use benzene.
- Identify and name the substituents.
- Number the ring to give the substituents the smallest possible number.
- Put the substituents alphabetically followed by the parent name.
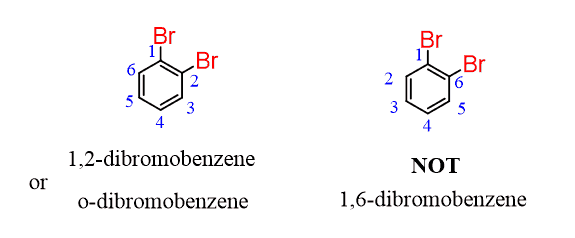
For example, in ortho-dibromobenzene, numbering from the top Br goes clockwise to have a 1,2, instead of 1,6 locant order:
We do the same way if there are more substituents:
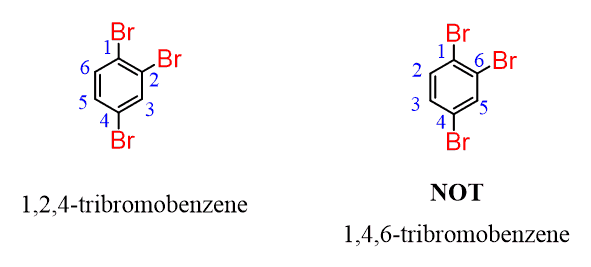
If the numbering does not make a difference, then give a smaller locant to the alphabetical priority:
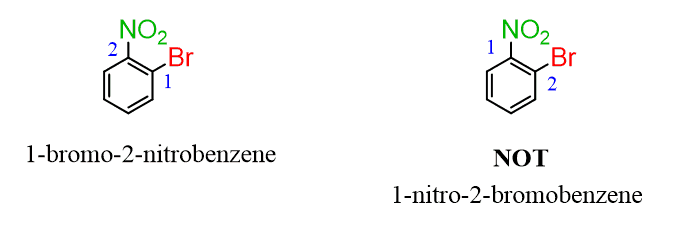
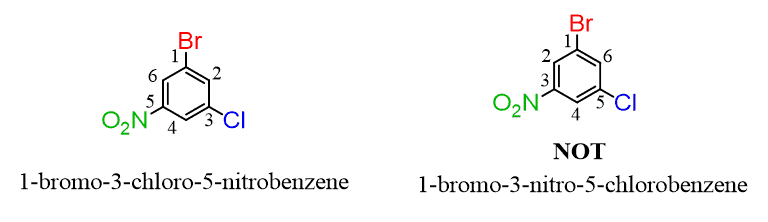
If there are more than two groups, and numbering doesn’t make a difference, start from the alphabetical priority and number the ring toward the next alphabetical priority:
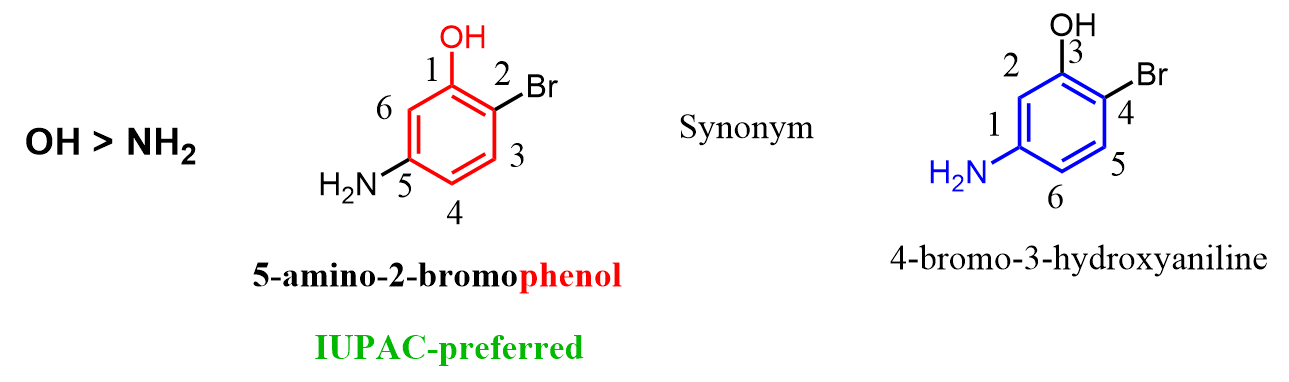
In reference to the question asked in the comments, I also wanted to address the situation when two common names are possible. For example phenol and aniline, we need to go based on the priority of the groups. In this case, we are comparing the amine and alcohol, and since OH stands above NH2 in the priority chart, the parent chain should be an alcohol (phenol) rather than aniline.

Check this 60-question, Multiple-Choice Quiz with a 1.5-hour Video Solution covering the naming and electrophilic aromatic substitution reactions.



it is very interesting websit but ,my email is not working on it.what is the problem.
Where’s the video for this?
Not all of them have a video, but you can check the quiz on aromatic compounds and also the summary quiz on the IUPAC nomenclature. They both have detailed video solutions.
I have a question about b). It has two common names, phenol and aniline. How do I decide which becomes the parent name? Thank you.
That’s a good question. These IUPAC tricks… From the IUPAC blue book (2013, page 64), it seems like we need to go based on the priority of the groups. In this case, we are comparing the amine and alcohol, and since OH stands above in the priority chart, the parent chain should be an alcohol (phenol) rather than aniline. So, the first option in the answer should be the correct one, although most often both will be acceptable and referred to as synonym names.
I really appreciate you!
Thanks so much!