We have seen that aromatic compounds are cyclic, planar and have a fully conjugated system of orbitals which gives them a special stability. There is, however, one more criterion that compounds must match in order to be aromatic. Not all the compounds that are cyclic, planar, and fully conjugated are aromatic.
For example, cyclobutadiene is a cyclic, planar, and fully conjugated but it is very unstable and can only be prepared at extremely low temperatures:

They are both similar in that they are cyclic, planar, and fully conjugated. The only difference is the number of π electrons and in order to be aromatic, the molecule must have a specific number of p electrons:
Aromatic compounds contain 4n+2 π electrons, where n is a whole number starting from 0. This is called the Hückel’s rule discovered by Erich Hückel in 1931.
For example, Benzene has 6 π electrons and it satisfies the Hückel’s rule since the n, in this case, is equal to one: Number of electrons = 4 x 1 + 2 = 6:

Unlike benzene, cyclobutadiene has only four π electrons and doesn’t satisfy the Hückel’s rule (if n=0, we get 2 e-, if n=1, we get 6 e–). The general formula for the number of electrons here is 4n (Möbius system) and this is the class of antiaromatic compounds which are highly unstable.

So one difference brings a new class of compounds that are structurally very similar to aromatic compounds, however, their properties and stability are on the exact opposite of the spectrum. These are antiaromatic compounds: cyclic, planar compounds with fully conjugated 4n π electrons.
To summarize, both aromatic and antiaromatic compounds are cyclic, planar, and fully conjugated and the only difference is the number of π electrons [Huckel (4n+2) – aromatic, Mobius (4n) – antiaromatic]:

Charged Aromatic and Antiaromatic Compounds
To demonstrate the contrast in stabilities of aromatic and antiaromatic compounds, let’s look at the following two reactions:
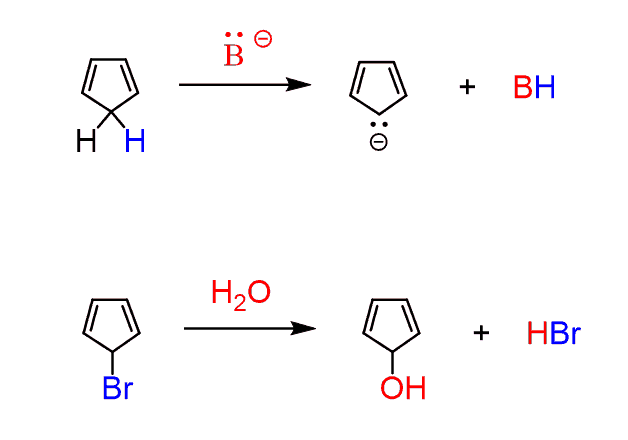
The first reaction is very fast compared to the deprotonation of other hydrocarbons (pKa 40-50). While deprotonation of allylic (next to C=C) C-H bonds requires the use of an organometallic (extremely strong base), cyclopentadiene can be deprotonated with a strong alkoxide (RO–) or a conjugate base of an amine. This indicates that the conjugate base of cyclopentadiene (cyclopentadienyl anion) must be more stable than regular carbanions:
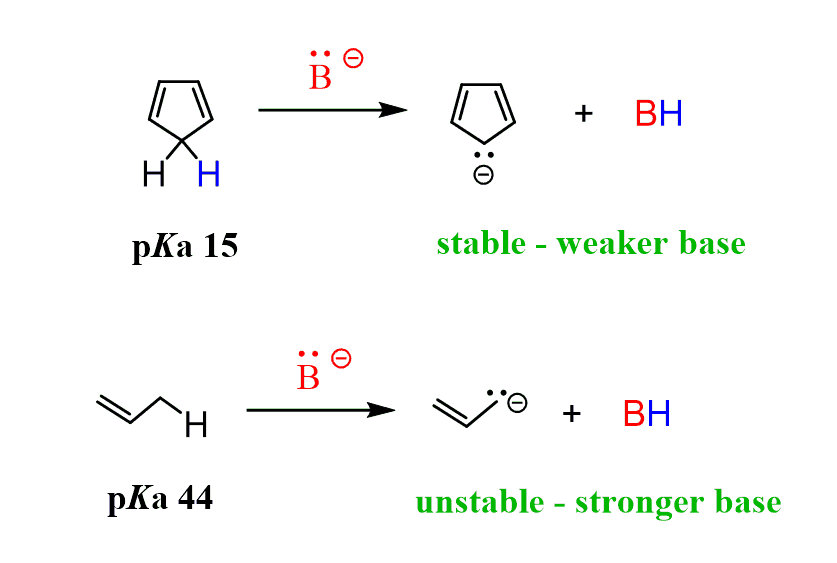
And indeed, the pKa of cyclopentadiene is 15 while allylic protons are generally at the 43-44 range. That’s enormously more acidic and it is only because the resulting cyclopentadienyl anion is aromatic.
So, let’s see how it is aromatic. It is clearly cyclic, but is it planar and fully conjugated?
The negatively charged carbon looks to be sp3-hybridized (steric number 3) which means the lone pair of electrons in the sp3 orbital cannot be delocalized since the sp3 orbital is not aligned with the p orbital of the π bond:

And here is something you want to remember, whenever possible, an atom will adopt a hybridization that supports forming of an aromatic system of electrons. In this case, the carbon changes the hybridization from sp3 to sp2 and the lone pair is now in the p orbital, fully delocalized over the ring.
This was about the stability of the cyclopentadiene anion explained by the fact that it is aromatic. Let’s now go back and figure out why the following reaction is unusually slow:
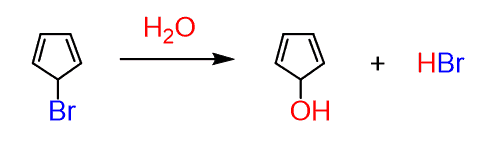
We have a weak nucleophile but can expect a fairly easy SN1 reaction since the loss of the leaving group bromine produces a resonance-stabilized allylic carbocation.
Why is it slower than the reaction of other secondary alkyl halides in SN1 reactions? It is even slower than the ones without getting a resonance-stabilized carbocation:
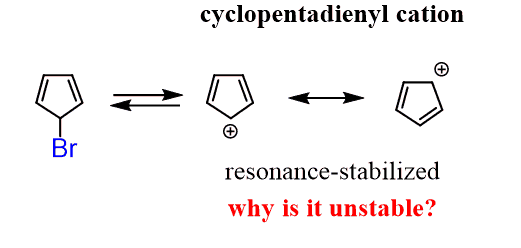
The answer is not related to the factors affecting the SN1 reactions such as the strength of the nucleophile, the hindrance, the leaving group and etc. It has to do with the topic of our discussion – stability of aromatic and antiaromatic compounds.
You can work on figuring it out before reading further.

The problem is that the cyclopentadienyl cation is highly unstable!
But we just said that the cyclopentadiene anion is very stable…
And isn’t that disturbing also because we always said that resonance-stabilized carbocations are “very” stable?
The reason for this is the number of electrons in the conjugated p orbitals of the cyclopentadienyl cation. It has 4 electrons which makes it antiaromatic (matches the 4n formula):

The empty p orbital of the carbocation helps to close the loop and make the system fully conjugated.
Now, compare this with the tropylium ion (cycloheptatrienyl cation) which is aromatic and very stable:
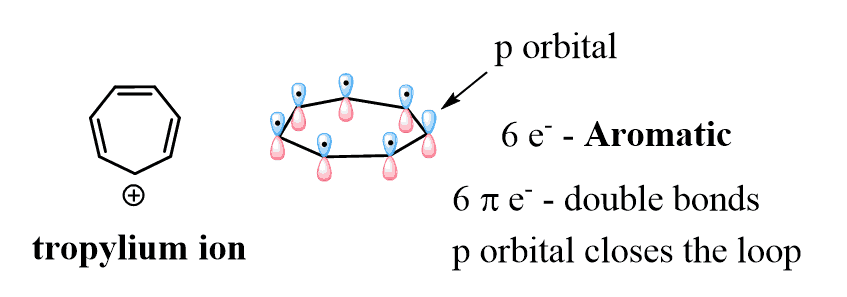
Unlike the cyclopentadienyl cation, it has 6 π electrons thus satisfying the Huckel’s rule, and again the loop of conjugated orbitals is linked by the empty p orbital of the carbocation.
Aromatic Heterocycles
Heterocyclic compounds are the ones containing atoms other than carbon in the ring system. For example, pyrindene is a common organic base used in many reactions.
It is an aromatic compound with 6 delocalized electrons thus satisfying the Huckel’s rule:
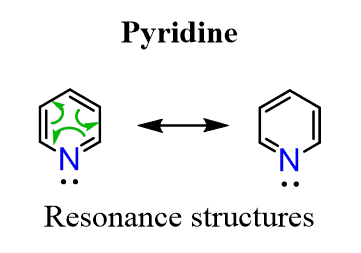
The question is how come the lone pair on the nitrogen is not part of the electron system changing it to 8 instead of 6 thus making pyridine antiaromatic?
Two things here:
1) The nitrogen is sp2-hybridized, and the lone pair of electrons is in a sp2 orbital which is perpendicular and out of the conjugated system:
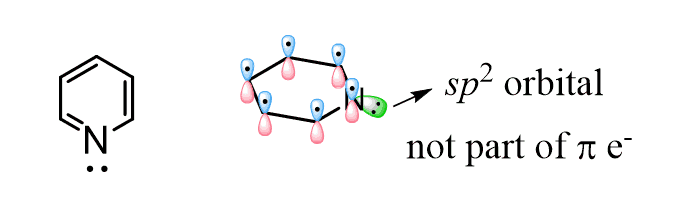
2) We mentioned this earlier; remember, whenever possible a given atom will adopt a hybridization state to avoid making the molecule antiromantic. Also, it will undergo a change to make the system aromatic whenever possible.
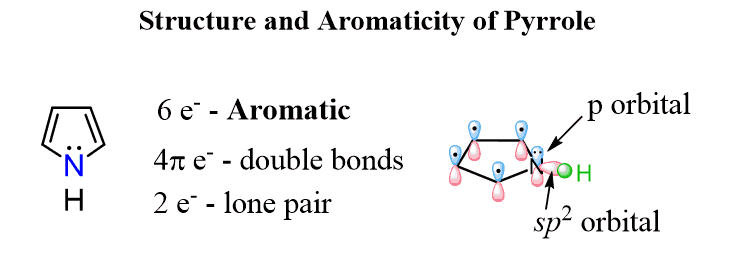
This can be shown on the example of pyrrole:
The nitrogen here looks to be sp3 based on the steric number (4 – 3 bonds and a lone pair) but because the molecule “wants” to be aromatic”, it changes the hybridization to sp2 thus placing the lone pair in a p orbital and forming a cyclic, planar, fully conjugated system of 6 electrons:
 Another good example is imidazole. It is found in an important biological amine histamine the excess of which is responsible for runny nose and watery eyes symptomatic of hay fever.
Another good example is imidazole. It is found in an important biological amine histamine the excess of which is responsible for runny nose and watery eyes symptomatic of hay fever.
What’s interesting about the structure of imidazole is that it combines then two principles we discussed above for favoring of aromaticity. It has one lone pair in a perpendicular to the conjugated system sp2 orbital which is not part of the aromatic system and another one in a p orbital because the nitrogen with single bonds is sp2 hybridized favoring the aromaticity. This, in total, makes 6 electrons of the aromatic system and one lone pair which is not part of that:
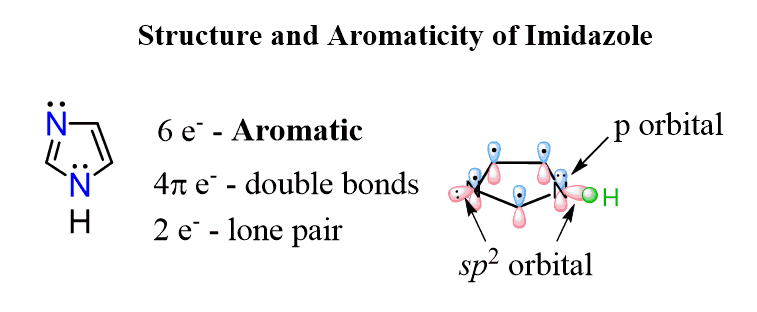
Imidazole and its derivatives are also used in organic synthesis serving as a base and nucleophile to speed up reactions such as the ones of alcohols with silyl ether for preparing protecting groups.
There are Also Nonaromatic Compounds
This post was about the importance of the number of electrons in conjugated systems and how it changes molecules from aromatic to antiaromatic based on the Huckel’s and Mobius rules.
Antiaromatic compounds being so unstable are very rare and as we discussed, whenever possible the molecule will “count or ignore” some electrons to be aromatic.
There is, however, one more class of compounds called not aromatic or nonaromatic and this, essentially, includes everything else that is not related to the aromatic and antiaromatic compounds. That’s a lot of compounds and we will talk about determining aromatic, antiaromatic, and nonaromatic compounds in the next post.
Check Also
- Naming Aromatic Compounds
- Introduction to Aromatic Compounds
- Benzene – Aromatic Structure and Stability
Identify Aromatic, Antiaromatic, or Nonaromatic Compounds - Frost Circle
- Annulenes
- Electrophilic Aromatic Substitution – The Mechanism
- The Halogenation of Benzene
- The Nitration of Benzene
- The Sulfonation of Benzene
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- Vilsmeier-Haack Reaction
- The Alkylation of Benzene by Acylation-Reduction
- Ortho Para Meta in EAS with Practice Problems
- Ortho Para and Meta in Disubstituted Benzenes
- Why Are Halogens Ortho-, Para- Directors yet Deactivators?
- Limitations of Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Arenediazonium Salts in Electrophilic Aromatic Substitution
- Reactions at the Benzylic Position
- Benzylic Bromination
- Nucleophilic Aromatic Substitution
- Nucleophilic Aromatic Substitution Practice Problems
- Reactions of Phenols
- Reactions of Aniline
- Meta Substitution on Activated Aromatic Ring
- Electrophilic Aromatic Substitution Practice Problems
- Aromatic Compounds Quiz
