From Acid-Catalyzed Hydration of Alkenes
In the previous post, we talked about the acid-catalyzed hydration of alkenes, which, in general, is a “Markovnikov” addition of water, giving the corresponding alcohol. A quick reminder, Markovnikov’s rule is followed when the X component of the HX reactant (for example, HCl, HBr, HI, HOH) adds to the more substituted carbon atom.

Let’s quickly recall the mechanism. The reaction starts with protonation of the double bond, forming the more substituted/stable carbocation. The consequent nucleophilic attack by water on this carbocation, followed by deprotonation, produces the more substituted alcohol as the major product.
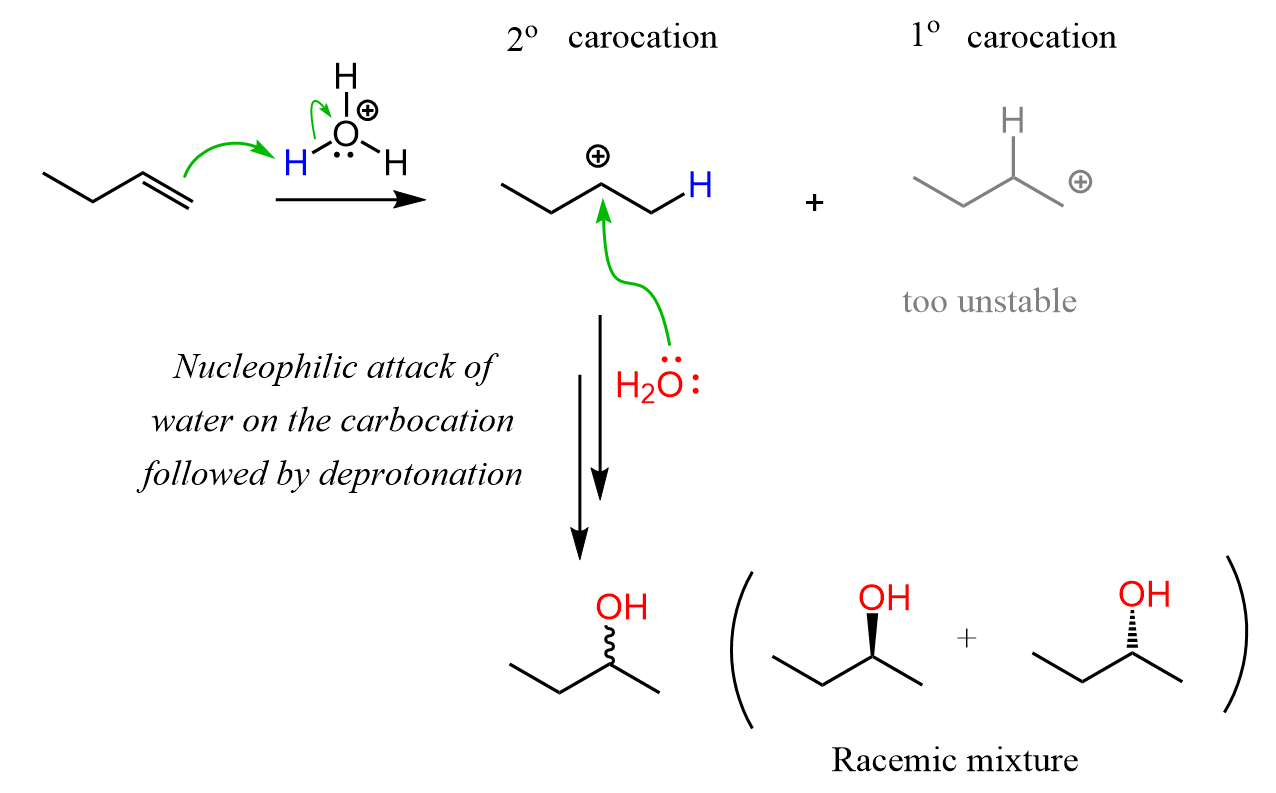
Remember, carbocation stability increases with the degree of substitution – the more stable carbocations are more stable. Therefore, in this example, the secondary carbocation is formed.

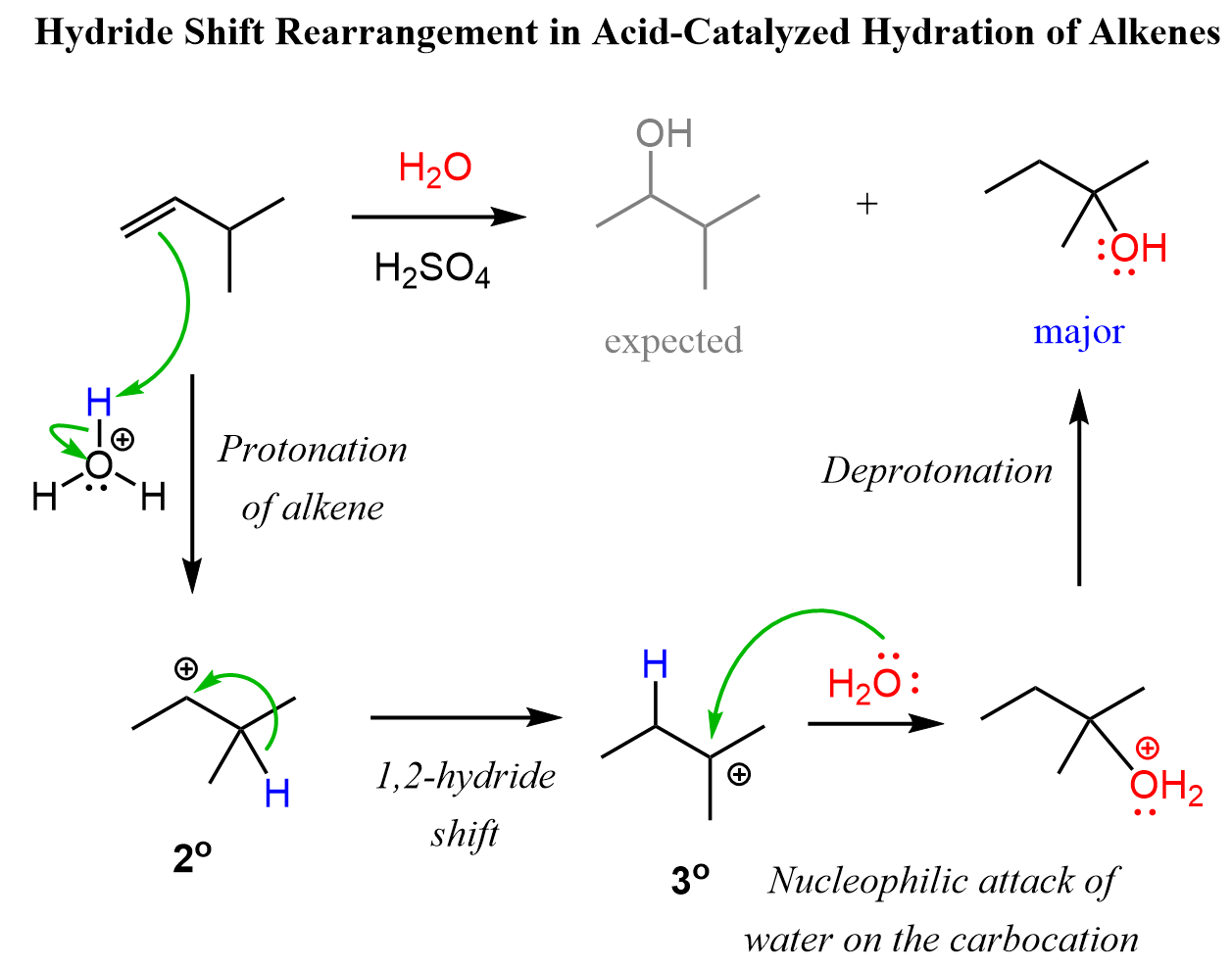
In the first sentence of this article, we mentioned that acid-catalyzed hydration generally follows Markovnikov’s rule. The reason for this is the possibility of carbocation rearrangements in electrophilic addition reactions of alkenes when they occur via an ionic mechanism. In the example above, when 1-butene is reacted with water, there is no possibility of converting the secondary carbocation to a tertiary carbocation; thus, no rearrangement can occur.
Let’s consider the addition of water to an alkene, going through a transformation of a secondary carbocation to a tertiary one:
Oxymercuration-Demercuration
To avoid possible rearrangements, an alternative method for the hydration of alkenes is used, known as the Oxymercuration-Demercuration or Oxymercuration-Reduction. In this method, mercury acetate (Hg(OAc)2) is used as a catalyst for the addition of water to alkenes:

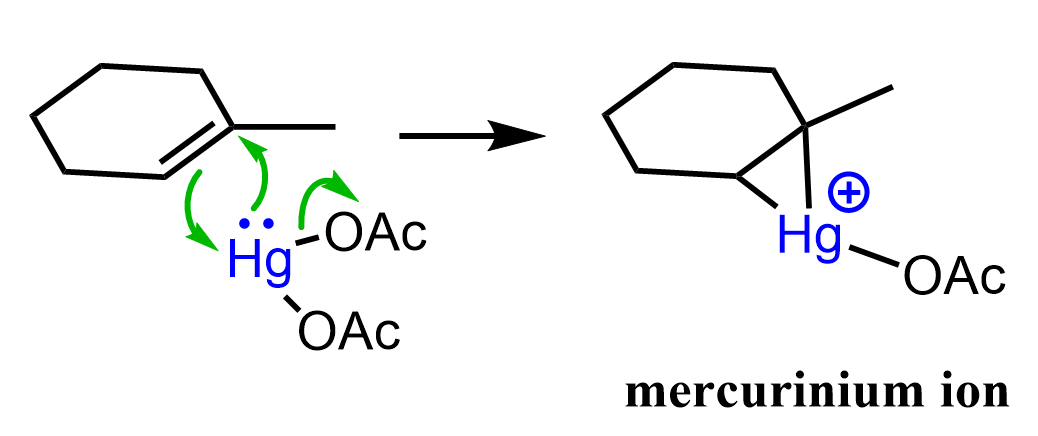
The idea behind using this catalyst is to alter the mechanism in a direction where the alkene becomes reactive enough for the nucleophilic attack of water (or other nucleophiles such as alcohols or amines) but not turned into a carbocation susceptible to rearrangements. This is achieved through a three-membered mercury-containing ring:
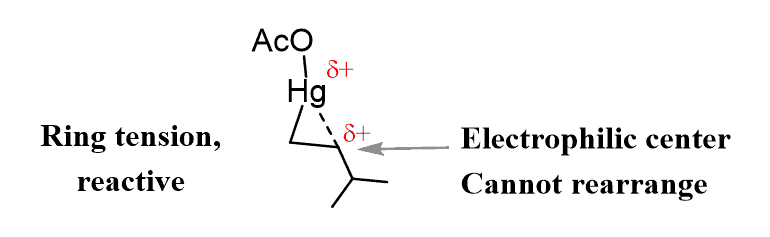
Before going into the details, here is one important thing to remember: three-membered rings are not very stable, meaning they are quite reactive due to ring strain, which is a result of the angle strain and torsional interactions.

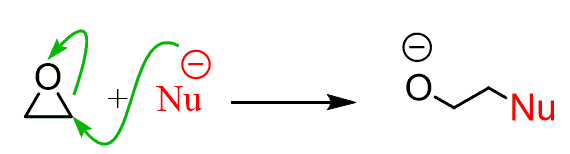
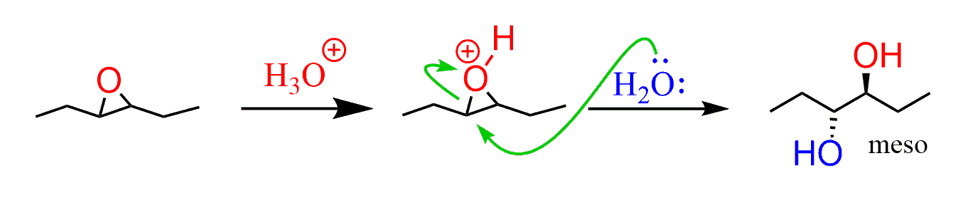
For example, epoxides are three-membered rings containing an oxygen, and when reacted with strong or weak nucleophiles, they readily undergo a ring-opening reaction:
This is how the anti dihydroxylation of alkenes occurs: they are first converted into an epoxide using a peroxy acid such as MCPBA, which is then opened up by water:
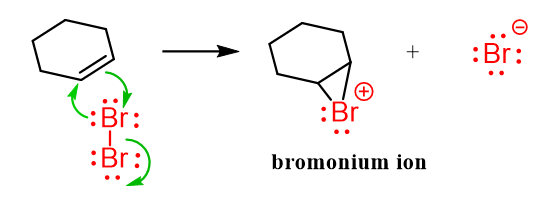
The pattern of reactivity in 3-membered cyclic structures can also be seen in the reaction of alkenes with halogens, where a reactive intermediate called halonium ion is formed, which is then attacked by either the halide ion or water:

And now, let’s see how the reactivity of epoxies and three-membered intermediates is related to the oxymercuration-demercuration reaction.
The Regiochemistry of Oxymercuration-Demercuration
As we have seen above, oxymercuration-demercuration is a regioselective reaction that follows Markovnikov’s rule. A reminder that regioselective means one, out of two or more, regioisomers is the preferred product of the reaction. For symmetrical alkenes, regioselectivity is not relevant because even if the structures look different, they represent the same product. The first reaction in the following set is an example of acid-catalyzed hydration and oxymercuration of a symmetrical alkene, and therefore, it gives the same product regardless of the method used for the hydration of the alkene.
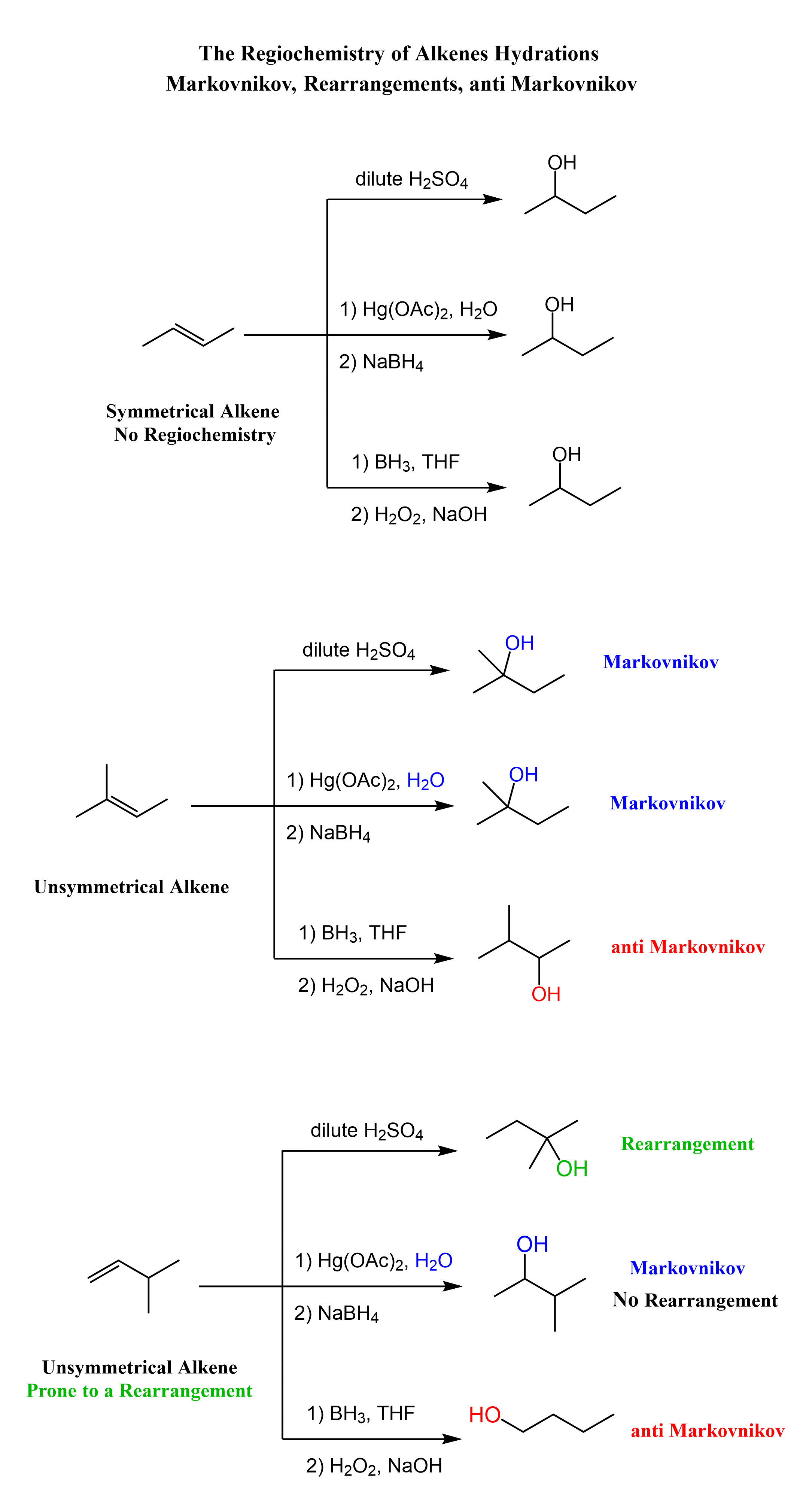
A quick reminder/heads up that BH3 with peroxide is used for anti-Markovnikov hydration of alkenes. The reaction is called hydroboration oxidation, and it places the OH on the less substituted carbon of the double bond:
The Mechanism of Oxymercuration-Demercuration
Let’s look at the mechanism of the Oxymercuration-Demercuration reaction and understand how it prevents possible rearrangement reactions.
In the first step, a positively charged cyclic intermediate called mercurinium ion is formed via the addition of mercury acetate to the double bond.
Once again, recall the formation of halonium ions during the halogenation of alkenes.
The mercurinium ion can be represented in different resonance forms depending on how the positive charge is distributed among the mercury and the carbon atoms.

These representations are not resonance structures in a classical definition since we break a single bond and are not moving electrons around. However, they do show how the electron distribution, thus the (partial) positive charge, is distributed between the atoms, so “resonance structures” is not too bad of a description for them.
We will see why these structures are important in explaining the regiochemistry of the reaction.
Being a positively charged three-membered ring, the mercurinium ion is very reactive and is immediately attacked by water in an SN2 fashion, opening up the ring. The key difference, compared to acid-catalyzed hydration, is that, unlike carbocations, the mercurinium ion does not undergo a rearrangement. What is also important is that the water attacks the more substituted carbon of the mercurinium ion, thus the corresponding alcohol is formed (Markovnikov addition):
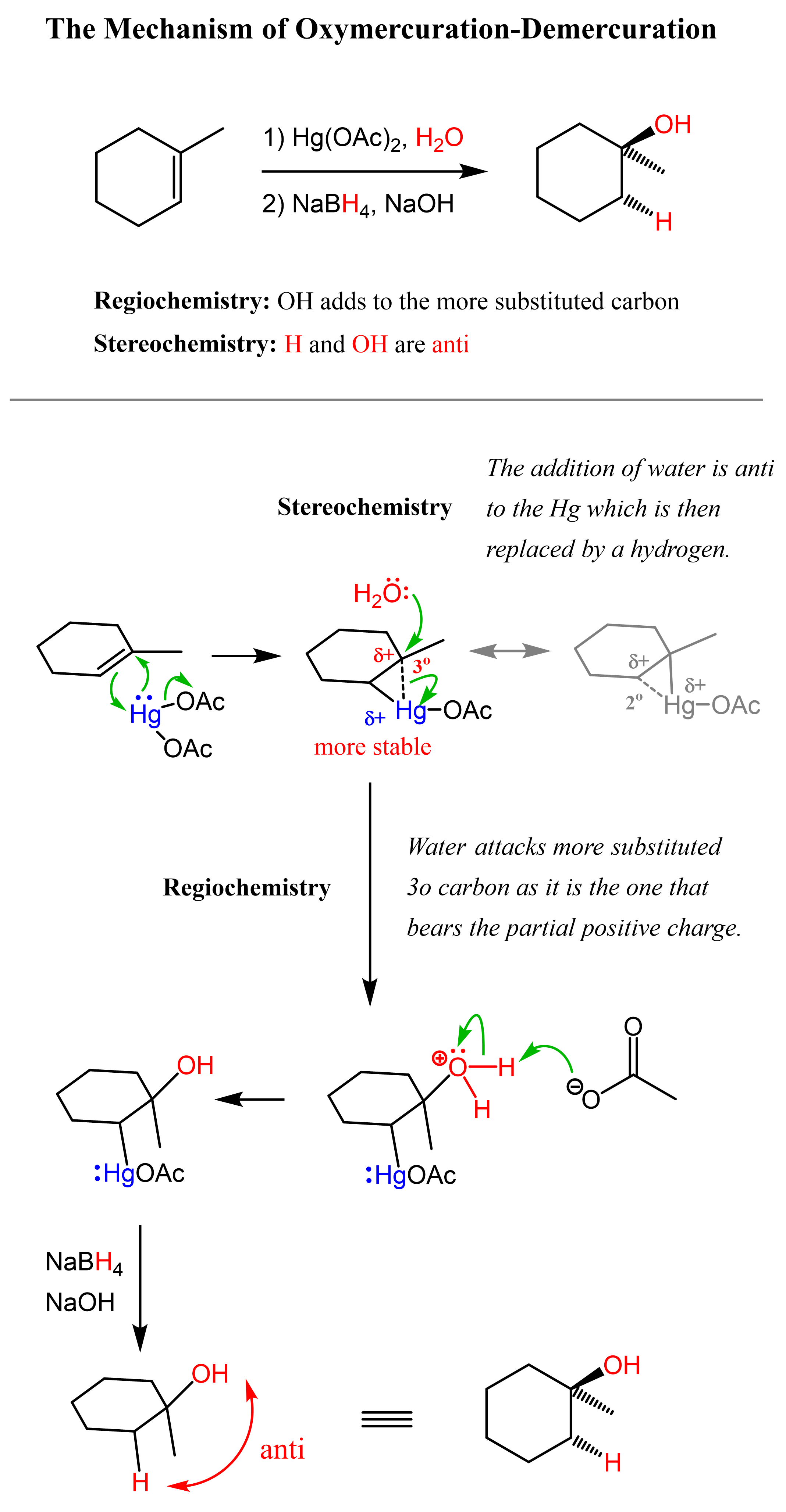
In the last part, the mercury is then removed using sodium borohydride (NaBH4), which reduces the acetoxymercury group and replaces it with its own hydrogen. This is described as demercuration, and it is believed to follow a radical mechanism that is not covered in undergraduate courses.
Why Does Oxymercuration-Demercuration Follow Markovnikov’s Rule?
Oxymercuration-Demercuration adds the OH group on the more substituted carbon atom of the double bond, and therefore, we can say that it is a Markovnikov addition of water to alkenes. The reason OH ends up being on the more substituted carbon atom is that the mercurinium ion is positively charged, and this charge is shared between the mercury and the two carbons in the ring. So we can draw three “resonance structures” which will show that the most optimal is when the partial positive charge is on the more substituted carbon atom because, remember, the stability of carbocations increases with the number of alkyl groups connected to the positively charged carbon atom:

As the indicated structure is the most stable, the mercurinium ion exists in a form where the more substituted carbon bears the partial positive charge, and therefore, water attacks it, defining the regiochemical outcome of the reaction.
The Stereochemistry of Oxymercuration-Demercuration
In oxymercuration-demercuration, the H and OH are added to the opposite faces of the double bond. This is called anti-addiction. With this said, there is generally no control over which face of the double the OH and H are added unless there are some steric interactions preventing the addition from one of the sides. Therefore, chiral centers (if any) are formed in equal amounts, and the product is a racemic mixture of two enantiomers.
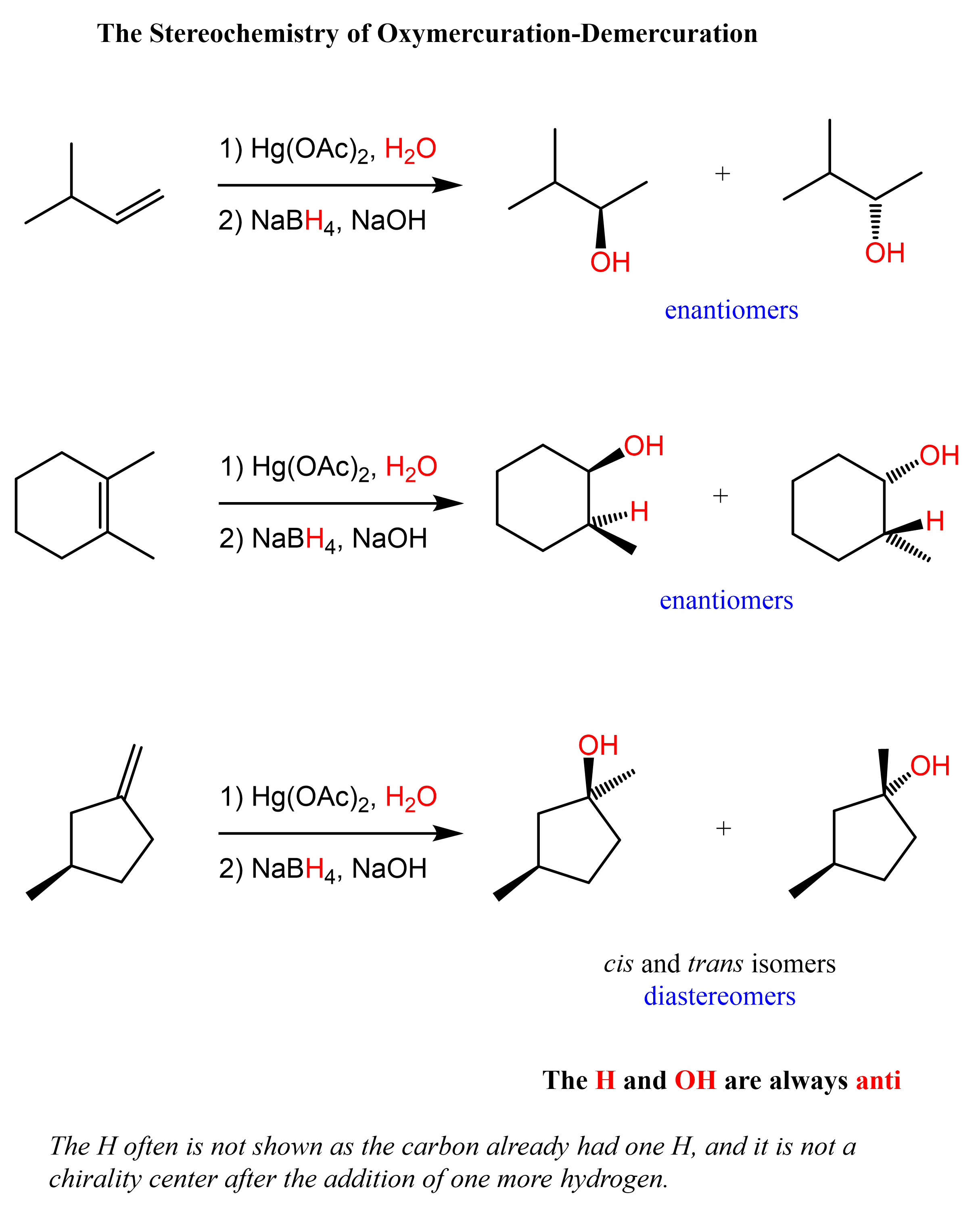
Notice from the last reaction that diastereomers can also be formed if the product contains two or more chirality centers. This is because while the newly forming centers are going to be R and S, the original chirality center is intact, thus the alcohols are not mirror images. Let’s also show the mechanism of how diastereomers can form in oxymercuration-demercuration reactions:
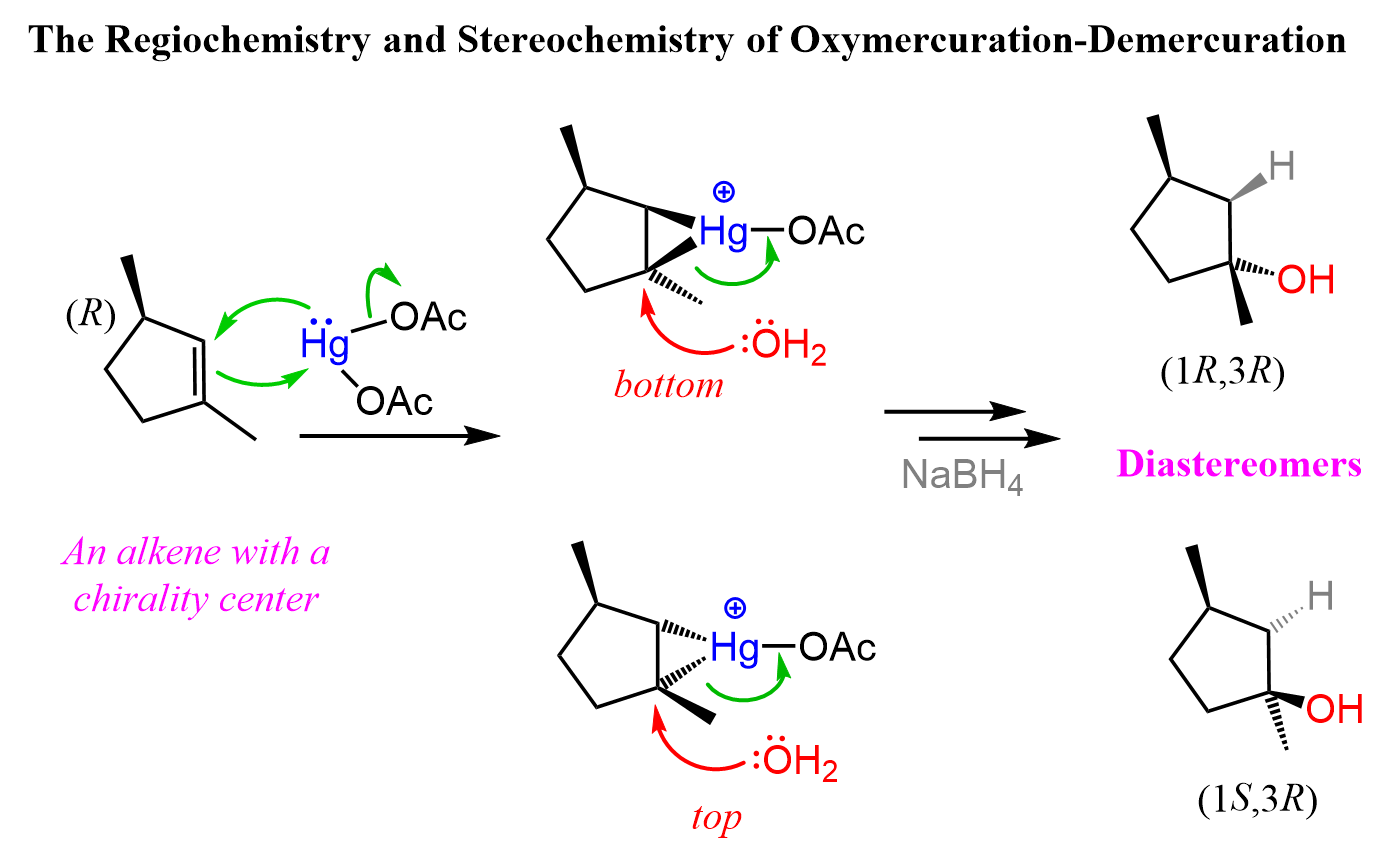
You can read more about the stereochemistry of the addition reactions to alkenes in this post.
Variations of Oxymercuration-Demercuration Reactions
The mercurinium ion can be used as an electrophile not only for water but for many other nucleophilic reactants such as alcohols, thiols, and amines. So, we can put together the following equation as a general guide for the Oxymercuration-Demercuration:
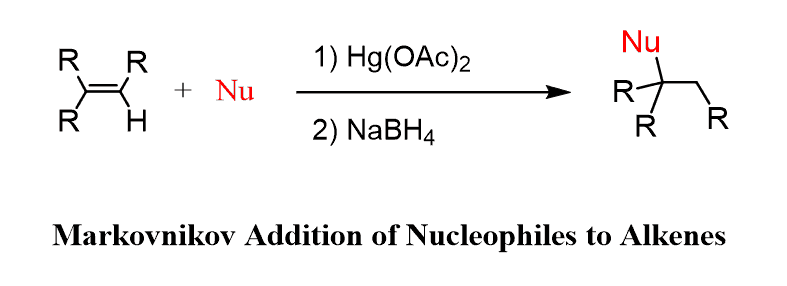
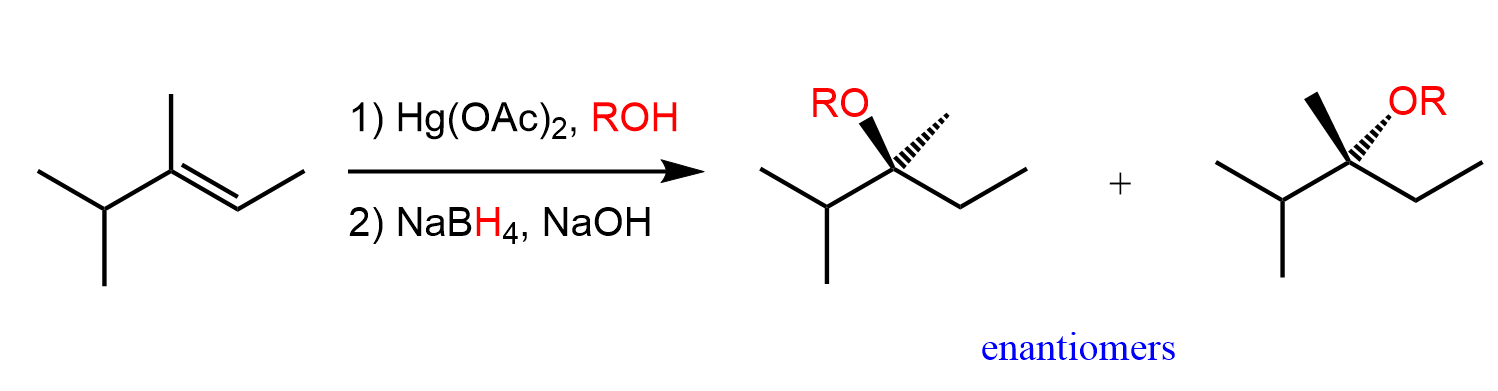
For example, the addition of alcohols to alkenes in the presence of Hg(OAc)2 is called alkoxymercuration, and it produces an ether following the same regio- and stereoselectivity of the reaction:

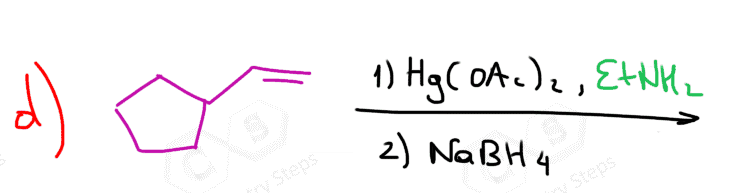

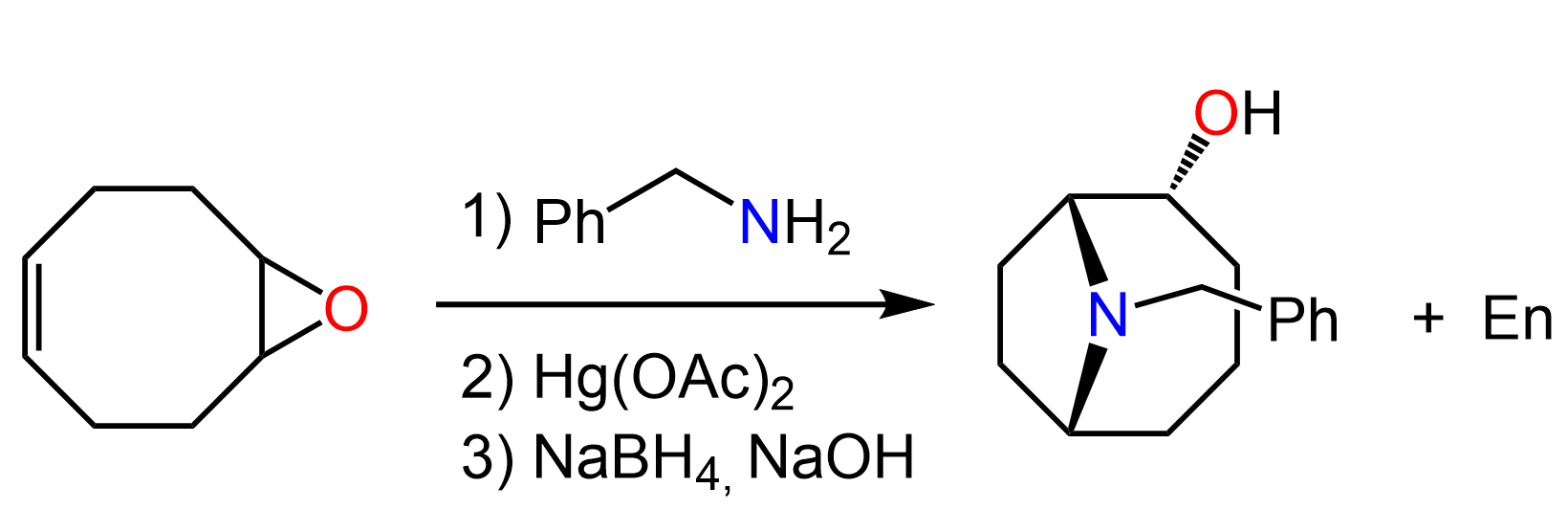
for question D, why would EtNH attach to the 2 prime carbocation, not the 1 prime?
Just like the water, any nucleophile used in this reaction attacks the more substituted carbon of the bridged mercurinium ion since it better stabilizes the partial positive charge.
The nucleophiles in Oxymercuration-Demercuration appear on the Markovnikov’s position
Just a clarifying question, is Oxymercuration-Demercuration the preferred method when it provides the same product as Acid-Catalyzed Hydration? I’m not understanding when you would choose Oxymercuration-Demercuration or Acid-Catalyzed Hydration.
Yes, they both follow the same regiochemistry, and the main reason for using oxymercuration is to prevent possible rearrangements that happen during acid-catalyzed hydration. Don’t forget to download the study guide on alkene reactions under the CS Benefits tab. There are also lots of quistions on this topic in the quiz on alkenes.
Let me know if you have any questions.
could u please explain the reduction from de oxymmercuration in detail