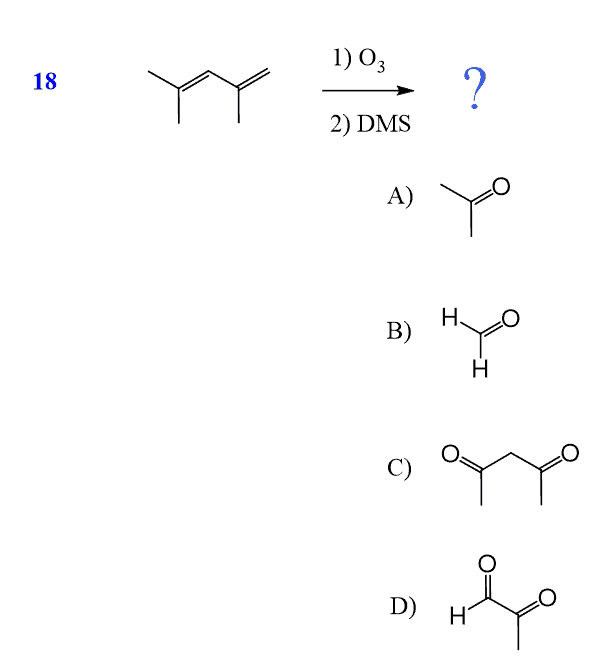
Ozonolysis is an efficient way of oxidizing alkenes (and alkynes as well) into their corresponding aldehydes and ketones:
One of the advantages of ozonolysis compared to other oxidative cleavage reactions is that it does not over-oxidize the alkene to carboxylic acid, unlike, for example, the potassium permanganate (KMnO4):

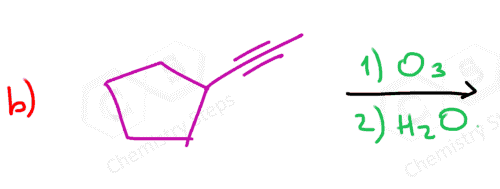
Even for terminal alkenes, it is possible to obtain formaldehyde instead of carbon dioxide, which is formed when strong oxidizing agents are used:

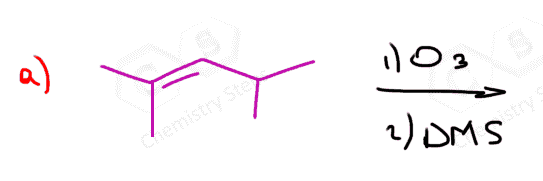
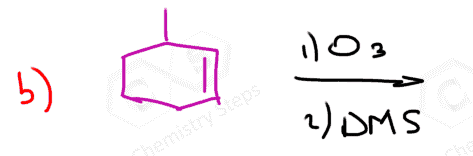
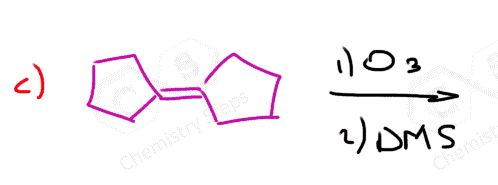
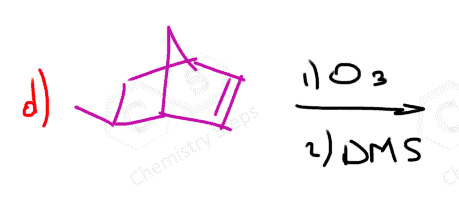
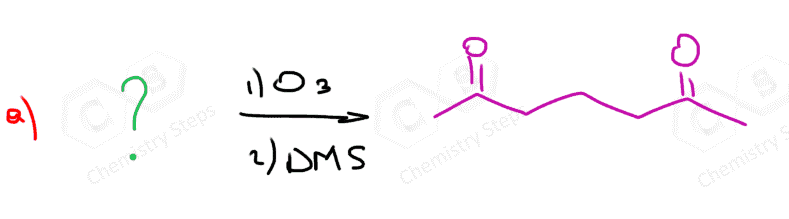
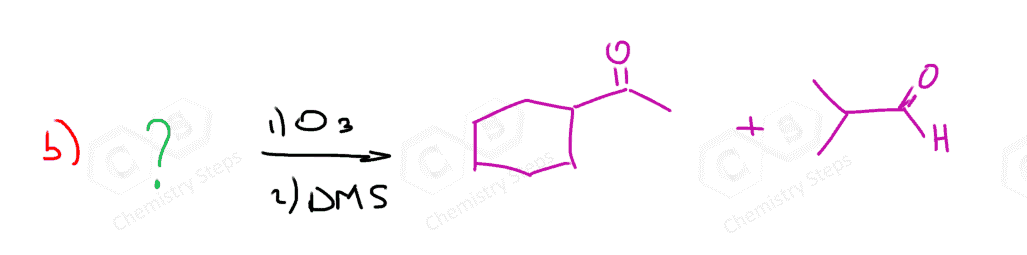
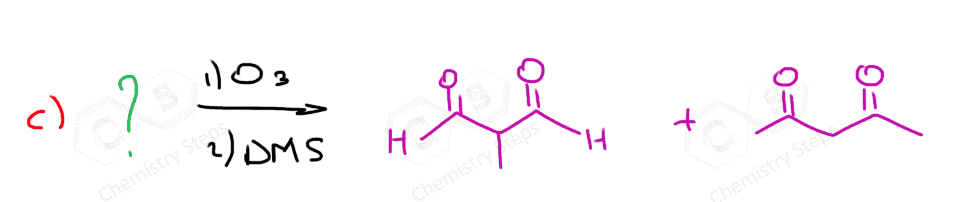
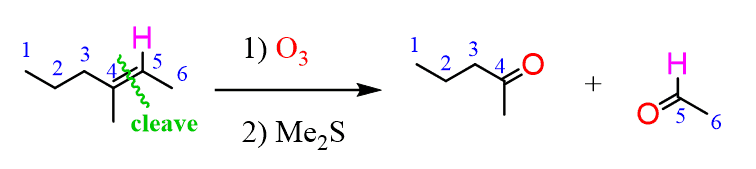
The same principle applies to cyclic compounds. Whenever you need to determine the product of an ozonolysis reaction, number the carbons (numbering carbons will help you a lot, no matter the exercises!) and replace the C=C double bonds with carbonyls:
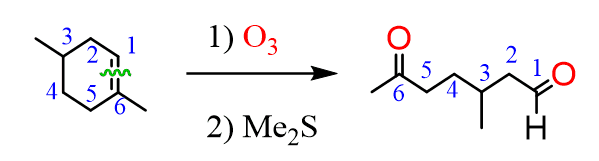
Ozonolysis Mechanism
The oxidation is achieved by bubbling ozone into the reaction mixture at low temperatures (-78 oC). The first step is the formation of a cyclic intermediate called molozonide (initial ozonide), which quickly rearranges into the more stable ozonide:

In the last step, a reducing agent, dimethyl sulfoxide (Zn and Acetic acid are also used) is used. Sulfur, being a polarizable atom, induces a partial positive charge on one of the connected oxygens and attacks it, cleaving the weak O-O bond:

As a result, the carbonyls are formed together with DMSO.
Retrosynthetic
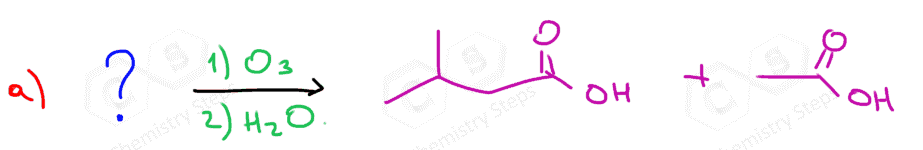
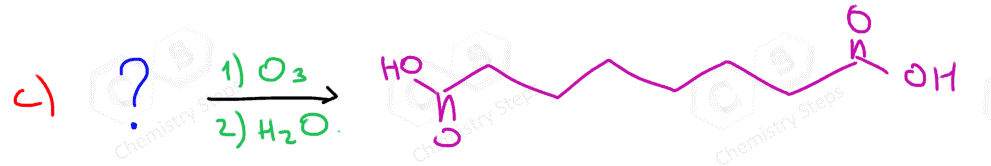
Ozonolysis can be used to determine the position of the double bond in an alkene:
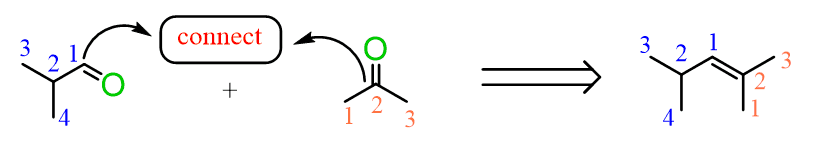
Connecting the two carbons of each carbonyl, we can visualize where the C=C double bond was in the initial alkene.
In the following Practice Problems, we will determine the products for ozonolysis of alkenes and alkynes, as well as retrosynthetic analysis to identify the starting alkene and alkyne in the ozonolysis reactions.