Changing the position of a double bond is a very important tool in organic synthesis. For example, let’s try to figure out what happens in this transformation:

The double bond has suddenly moved to produce a new regioisomer of the starting material. This may look confusing at first but we are going to break it down to simple steps in a very intuitive strategy.
To achieve this, we use the regioselective addition to alkenes (Markovnikov’s rule) and the regioselective elimination reactions based on the Zaitsev and Hofmann rules.
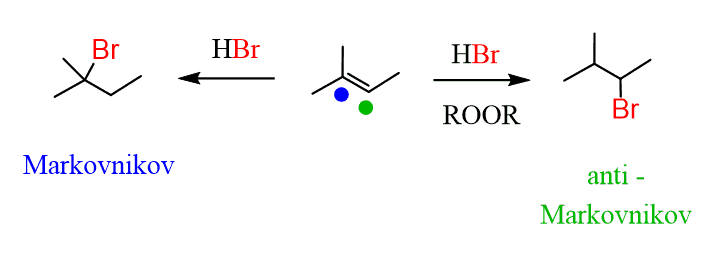
A little reminder on these: You can selectively add a halogen to the most and least substituted positions of a double bond:
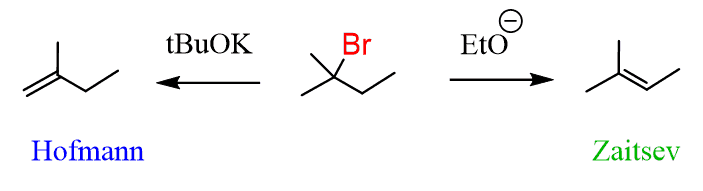
You can also selectively introduce a double bond by E2 reactions using a non-hindered (Zaitsev) and a bulky (Hofmann) base:
So, here is what you can do when asked to move the double bond around.
- Indicate the direction where the double bond is moving:
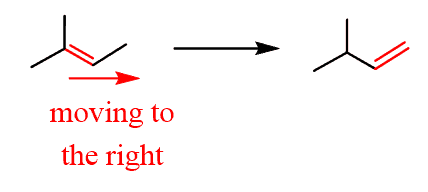
2. Add a halogen to the correct carbon based on the direction. In this case, it is the anti-Markovnikov position, so you need a radical hydrobromination:
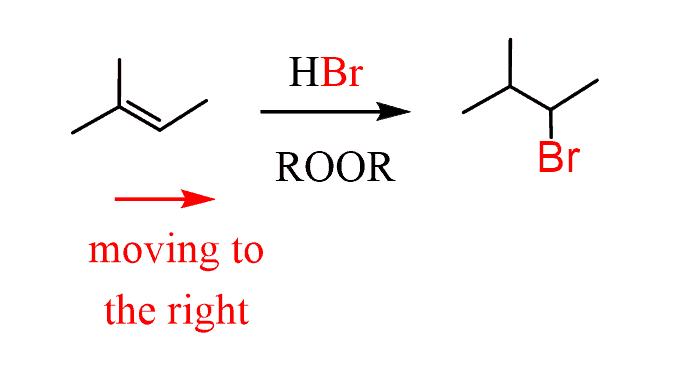
- Perform an elimination – still moving to the right. This means we need a sterically hindered base:
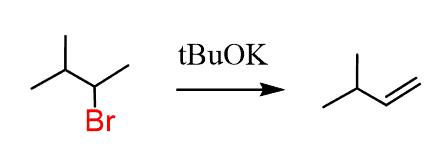
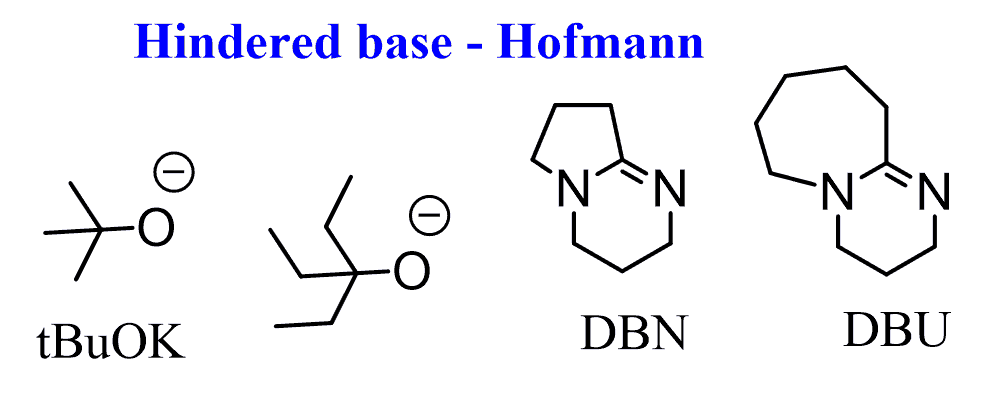
Some commonly used sterically hindered baes are:
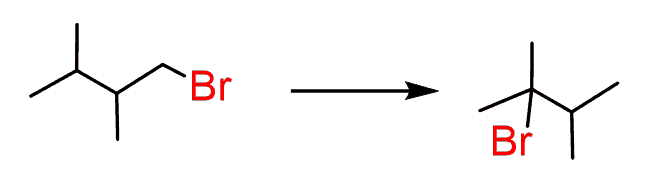
This is how the double bond can be moved by using a cycle of regioselective addition and elimination reactions and the same principle is applied to moving the position of a leaving group:
More details of this are in the next post – Changing the Position of a Leaving Group


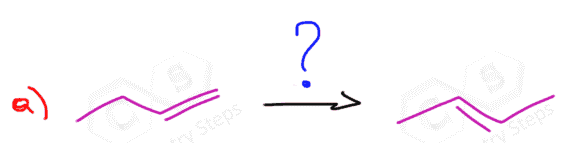







When we add HBr to the molecule to break the alkene, should we look for the rearrangement? Thank you.
Yes, you need to always look out for rearrangements whenever a carbocation is formed. I discussed this, for example, in “Acid-catalyzed hydration of alkenes“.
You can also check the quiz on alkene addition reactions as well.
Thank you for the confirmation.