The Stereochemistry of Alkene Addition Reactions
In the previous post, we talked about the Markovnikov’s rule and learned that in the addition reaction of HX to an unsymmetrical alkene, the H adds to the carbon that already has the greater number of hydrogen atoms. Or, looking from the perspective of the X group, we can say that the X atom (or group) adds to the carbon that already has the greater number of carbon atoms:

This is the regiochemistry of the reaction as it shows why one regioisomer (constitutional isomer) is formed preferentially over the other one.
Sometimes the addition to the alkene results in a product with one or two stereogenic (chirality) centers. The resulting product can be a mixture of stereoisomers which can be enantiomers or diastereomers.
At this point, it might be a good idea to refresh the concepts of constitutional and stereoisomers by following the corresponding links above. Additionally, you want to check some practice problems identifying molecules as Enantiomers Diastereomers the Same or Constitutional.
Without going too far, let’s look at the product in the reaction we just talked about. It has a chirality center and therefore can exist as two enantiomers:

The question is which one forms in excess, or in other words what is the stereochemistry of this, and in general, for the electrophilic addition reactions of alkenes. And this what we will discuss in today’ post.
Addition Reactions that Form a Product with One Chirality Center
There are two possible scenarios for the starting alkene that we can have;
1) the starting material alkene has no chiral centers
2) there is a chiral center in the alkene.
Let’s start with the first option.
Addition to an alkene with no stereogenic center
For example, draw the major product(s) of the following reaction:
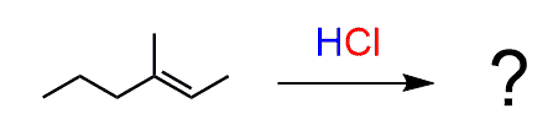
For the regiochemistry, we know that according to the Markovnikov’s rule, the Br will be added to the more substituted carbon:

Notice that the carbon where the Cl had added became a chiral center, therefore we need to address whether it has an R or S absolute configuration. And the answer is that it actually is a racemic mixture of R and S enantiomers.
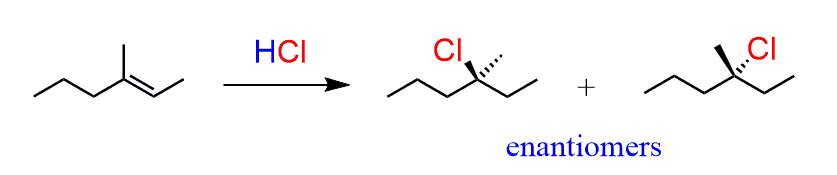
This is explained by the fact that carbocations are sp2-hybridized, flat centers (we are talking about the positively charged carbon) and the nucleophilic attack occurs from both sides:
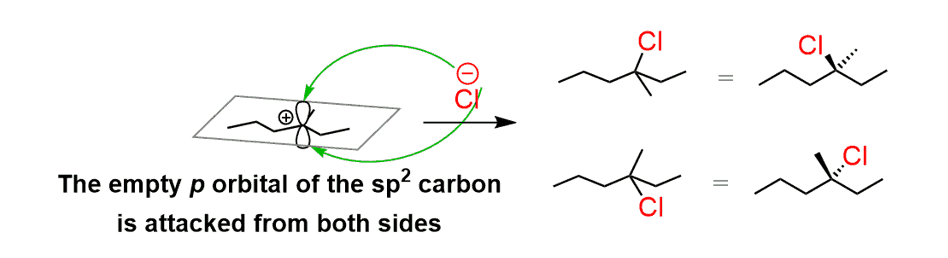
This attack happens in the same amounts and as a result, a racemic mixture of two enantiomers is obtained.
- To summarize, addition reactions of alkenes with no stereogenic center that form a product with one stereogenic center produce a racemic mixture of enantiomers.
Scenario 2 – Addition to an alkene with a stereogenic center
If the starting alkene contains a chirality center, and the addition to the double bond creates a new chirality center, then the products are diastereomers:

The asymmetric center in the starting material is not changed since it does not participate in the reaction. The new asymmetric center, however, is opposite for each product depending on the face the bromide had attacked the carbocation.
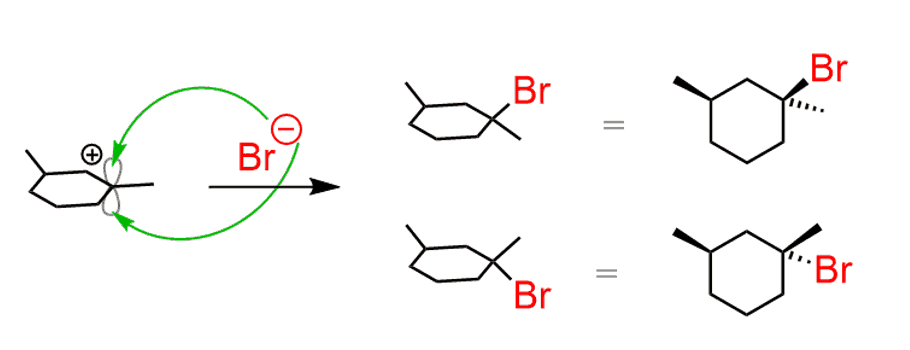
- Therefore, the products are a mixture of diastereomers. Similar to this, SN1 reactions can also produce diastereomers even though we usually say that they give a racemic mixture. You can check problems 3.5 and 3.6.
Addition Reactions that Form a Product with Two Chirality Centers
Let’s consider the reaction of 1,2-dimethylcyclohexene with HBr. The starting material does not have any asymmetric centers. However, it produces four stereoisomers in this reaction!

Let’s see how this happens and what is the relationship between these stereoisomers. The first step is, as usual, the protonation of the double bond and what is important here is to remember/visualize that the H can add from both faces of the double bond:

Notice that because of the hydrogen adding from different faces, the new chirality canter can be either R or S, and statistically it forms in a 50:50 ratio. There is no preference as to hydrogen adding from one side or the other side – no stereoselectivity.
Similar to this, once the carbocation is formed, the bromide ion attacks the positively charged, trigonal planar carbon from above or below. This variety of additions results in four stereoisomers as final products.

These are pairs of enantiomers and each enantiomer has two diastereomers:

There is no control on the stereochemistry of this reaction and the proton, as well as the bromide (or any other substituent), add from both sides in equal amounts. Therefore, the reaction is not stereoselective i.e. none of the stereoisomers is formed preferentially. It is also not stereospecific, as the same products are obtained regardless if the cis or trans stereoisomers of the alkene are used.
- Therefore, when two stereogenic centers are formed in an addition reaction of alkenes, all the possible stereoisomers are formed, and the product is a mixture of enantiomers and diastereomers.
Syn and Anti Additions to Alkenes
Labeling the addition as “Above” and “Below’ is not scientific and not accurate either as the direction depends on the viewer. Therefore, a more universal approach is used to describe the stereochemistry of additions to the double bond. When two groups add to the same side of the double bond, it is called a syn addition and when they add from different sides, it is an anti addition.

A representative example of a syn addition to alkenes would be the hydroboration-oxidation reaction where the H and OH groups are adding to the same side of the double bond. The reaction, however, is going with a different mechanism; there are no ionic intermediates and it is a concerted mechanism:
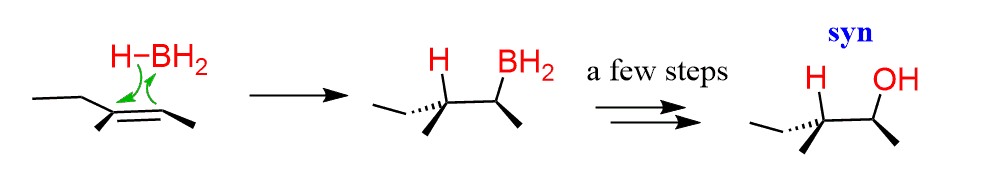
And a good example of an anti addition is the dihydroxylation reaction by peroxyacids.

Notice how the H and OH groups appear on opposite sides in the product.
These, of course, are not the only examples of syn and anti addition reactions to alkenes. You can find more examples from the list below.


Can you please explain more about d? Can a molecule be diastereomers without a chiral center? Thank you.
We normally say that diastereomers have at least one chiral center where the R, S configuration is the same i.e. it is R, R or S, S, and all the others are inverted, or, all of them are the same except one that is inverted. In other words, it can have all the chiral centers inverted because that makes two molecules enantiomers, and if they are all the same, then it is the same compound.
This brings a common question of whether diastereomers always have chiral centers, or, are they always chiral.
And the answer is no, they do not necessarily have chiral centers. The best example of this is the cis and trans or E and Z isomerism.
Because the connectivity of atoms is the same and the arrangement is different, these are stereoisomers. Specifically, because they are not mirror images, we classify them as diastereomers. So, cis and trans isomers are diastereomers.
Going back to the absolute configuration, we can say that if the diastereomers are chiral, then the statement about the R and S configuration is correct. However, if they are not, then it is irrelevant to talk about the R and S configuration and chirality in general – they are achiral diastereomers.
Thank you for the detailed explanation! It really helps.
Very welcome.