Many addition reactions to alkenes are stereoselective since one (set) of stereoisomers is formed from a starting material that is not stereoisomeric. For example, the bromination of cyclohexene forms two enantiomers of trans-1,2-dibromocyclohexane. The cis-dibromo isomer is not formed, and we can say that the reaction selects/chooses to form one set of stereoisomers over the other one.

This is a result of the mechanism going by anti addition to the double bond. Again, it could have gone by a syn addition, but it chooses to do an anti addition. Therefore, it is a stereoselective reaction.
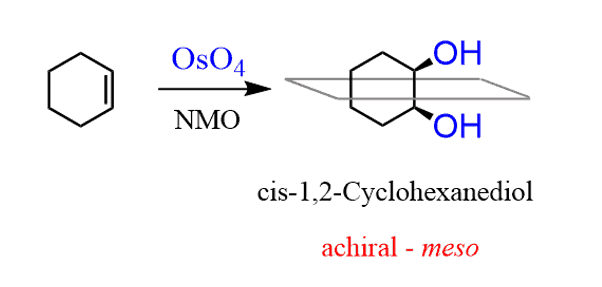
A meso compound is formed when the cyclohexene undergoes a syn addition. For example, syn hydroxylation produces the cis-dihydroxycycloheaxane, and the presence of a symmetry plane makes it a meso compound:
What is tricky is that sometimes, you may see a reaction going through an anti-addition mechanism forming a cis product – both groups pointing in the same direction:

It looks like the chlorination of the alkene had occurred as a syn addition, but we know the mechanism, and we know that it is an anti addition:
*Note: The term syn is more appropriate for describing a system where a rotation about a single bond is possible, while cis is used mostly for alkenes and cyclic systems, which are locked and cannot change the orientation of the groups. However, in this article, the terms syn/cis and anti/trans are used interchangeably. Sorry – a habit.
So, what is going on here?
You need to remember that sometimes the stereochemistry of the product depends on the stereochemistry of the starting alkene. I.e., each stereoisomer forms a given set of stereoisomers – the reaction is stereospecific.
Therefore, if we start with the trans-2-butene, then the product, drawn in a zig-zag, will have the Cl’s as a wedge and a dash.
The cis-2-butene also undergoes an anti chlorination, but in order to show the product as a zig-zag, we need to rotate around the sigma bond, which puts the Cl’s on the same side (two wedges or two dashes):

And that is why it looks like the chlorination had occurred by a syn addition. The first reaction with the cis alkene forms two enantiomers, while the second one gives only one trans isomer as a product.
Notice that the product of the trans alkene is achiral since it is a meso compound.
Syn and Anti additions and organic synthesis
This problem involves a reaction from alkynes. However, it is still useful if you haven’t talked about it in your class.
Suppose you need to propose reagents for the following two syntheses:
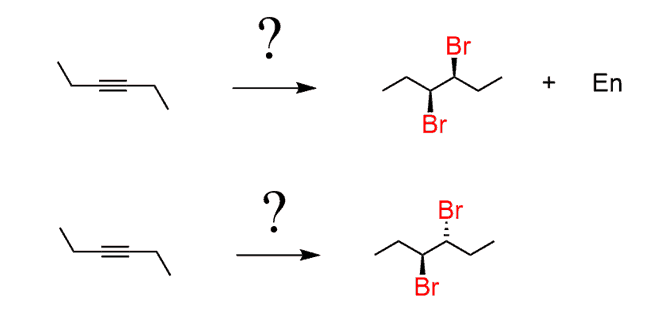
Notice that in the second reaction, there is no enantiomer mentioned and it is because the product is a meso compound.
First, you need to realize that vicinal dihalides are prepared from the halogenation of alkenes. So, putting this in a retrosynthetic scheme, we can visualize that the cis enantiomers must have come from the corresponding cis alkene and the trans enantiomers are synthesized from the trans enantiomer, as we have discussed above:

Cis and trans alkenes can selectively be prepared by the hydrogenation of alkynes using Lindlar’s and metal-dissolved reductions, respectively. Therefore, the following sequence of reactions would be suitable to prepare the desired stereoisomers of the haloalkanes:

Check the syn and anti addition reactions below, as they also have associated practice problems to master this concept:
Check Also
- Electrophilic Addition Reactions to Alkenes
- Markovnikov’s Rule
- Markovnikov’s Rule with Practice Problems
- Addition of Water to Alkenes
- Acid-Catalyzed Hydration of Alkenes with Practice Problems
- Rearrangements in Alkene Addition Reactions
- Oxymercuration-Demercuration
- Addition of Alcohols to Alkenes
- Free-Radical Addition of HBr: Anti-Markovnikov Addition
- Hydroboration-Oxidation: The Mechanism
- Hydroboration-Oxidation of Alkenes: Regiochemistry and Stereochemistry with Practice Problems
- Halogenation of Alkenes and Halohydrin Formation
- The Regiochemistry of Alkene Addition Reactions
- The Stereochemistry of Alkene Addition Reactions
- Ozonolysis of Alkenes with Practice Problems
- Syn Dihydroxylation of Alkenes with KMnO4 and OsO4
- Anti-Dihydroxylation of Alkenes with MCPBA and Other Peroxides with Practice Problems
- Oxidative Cleavage of Alkenes with KMno4 and O3
- Alkene Reactions Practice Problems
- Changing the Position of a Double Bond
- Changing the Position of a Leaving Group
- Alkenes Multi-Step Synthesis Practice Problems
- Alkene Addition Reactions Practice Quiz
- Reactions Map of Alkenes

