In this post, we will talk about the Grignard reaction – a fundamental reaction in organic chemistry discovered by Victor Grignard in 1912, which gave him the Nobel prize award.
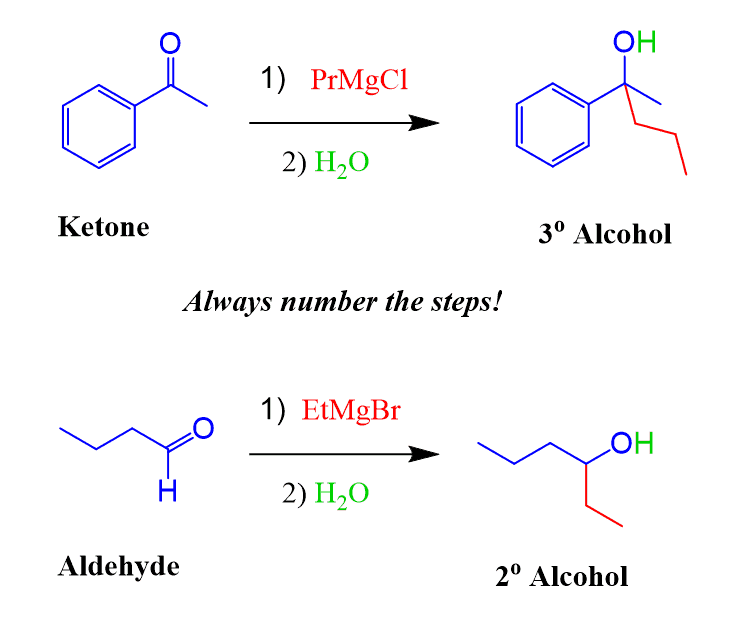
The most common and important Grignard reaction is the one with compounds containing a carbonyl group. Grignard reagents react with aldehydes, ketones, and ester to form alcohols. Aldehydes and ketones form secondary and tertiary alcohols respectively, while esters are reacted with an excess Grignard reagent to produce tertiary alcohols:

Grignard reagents can also react with acid chlorides, anhydrides, nitriles and epoxides. These reactions are slightly different so let’s first discuss the principle of the Grignard reaction.
Grignard Reaction Mechanism
Let’s discuss the mechanism of the Grignard reaction by starting with aldehydes and ketones. The origin of the Grignard reaction is the great imbalance of electron distribution. On the one hand, we have the C-Mg non-metal-metal polar bond which is almost ionic, and on the other hand, we have the C=O bond where the electron density is on the oxygen and the carbon is highly electrophilic:

When these two are mixed, the strongly Grignard reagent uses the C-Mg electron pair to form a bond to the carbon atom of the carbonyl. This nucleophilic attack pushes the electron pair of the carbonyl π bond to the oxygen forming an alkoxide. The resulting alkoxide ion associated with Mg2 and the halide is then neutralized by reacting with water or an acid:

At this point,t it is important to mention that Grignard reagents are very strong bases. The high electron density on the C-Mg carbon atom makes it not only a good nucleophile but also a strong base.
This brings up a very important rule that you need to always follow when working with a Grignard reagent or any other organolithium. And that is making sure the reaction is carried out in dry conditions – no trance of water should be present because it will react with the Grignard before the nucleophilic attack happens
This essentially kills the Grignard reagent and the reaction won’t work:

This restriction is not only applied for water but also for any compound that contains a proton, acidic enough to react with the Grignard reagent. That can be, for example, an alcohol or a carboxylic acid.
You may want to check this post about the protonation-deprotonation principle in acid-base reactions.
Nevertheless, sometimes it is needed to prepare a Grignard reagent that contains an alcohol group or react it with a carbonyl that contains an OH group:
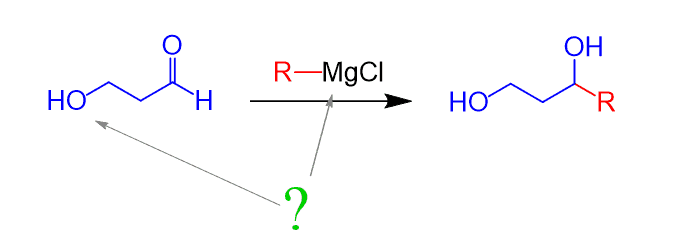
What you need to remember is that these two groups are incompatible, as they will react immediately. To prevent this undesired reaction, protecting groups of alcohols are used which is covered in this post.
Therefore, it is crucial to add the water (or acid) after the Grignard reagent has already reacted with the electrophile. The reaction must be shown in two steps– first, the nucleophilic attack of the Grignard and then the acidic work-up:
Grignard Reaction with Esters
Grignard reagents also react with esters to produce tertiary alcohols by a similar mechanism to the aldehydes and ketones:
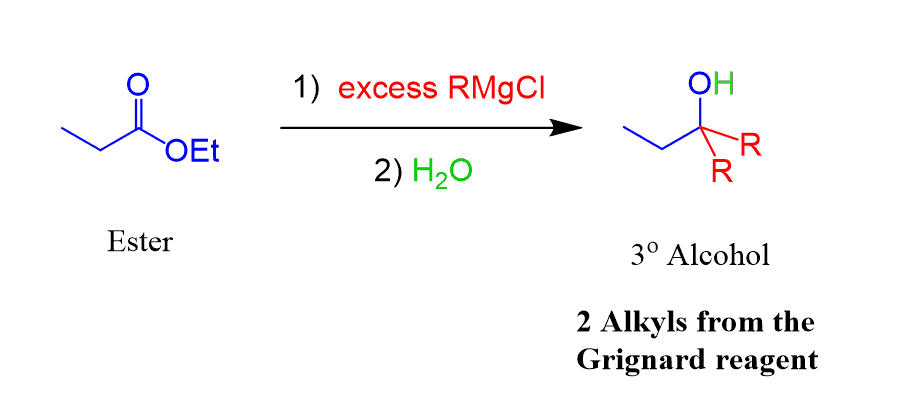
However, there is one important difference! Esters react with two equivalents of Grignard, forming a tertiary alcohol.
Let’s see how this happens.
In the first step, we have the nucleophilic attack of the Grignard making the C-C bond and shifting the electrons of the π bond to the oxygen.
The difference between aldehydes and ketones is that the product of this addition reaction to the carbonyl contains an alkoxy group on the tetrahedral intermediate. This group is a relatively good leaving group (more stable than the Grignard reagent) since it is a weaker base than the alkyl groups on the tetrahedral carbon. And the lone pairs of the oxygen can move down to expel this group and restore the carbonyl π bond:

This forms a ketone which are more reactive than esters since the electrophilicity of the carbon atom of the ester is partially suppressed by the lone pair of the oxygen through resonance stabilization:

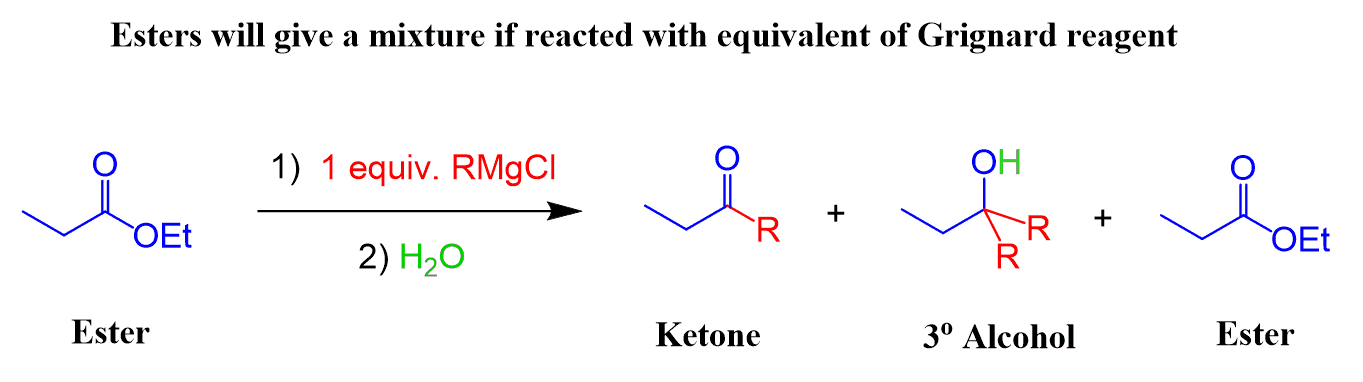
After the first addition to the carbonyl, we have the Grignard reagent, ester, and a ketone in the reaction mixture. The ketone reacts further to form a tertiary alcohol, as we have seen earlier. In addition, the ester, being in a much higher concentration, still reacts with the Grignard as well.
This demonstrates why two equivalents of Grignard are needed when reacted with esters.
If one equivalent was used, a mixture of a tertiary alcohol, ketone, and unreacted ester would have been obtained after the acidic work-up:
Grignard Reaction with Nitriles
Nitriles contain a polar carbon-nitrogen triple bond. Remember, in a triple bond we have two π bonds, and this makes it a great candidate for reacting in a Grignard reaction. The product of this reaction is a ketone:
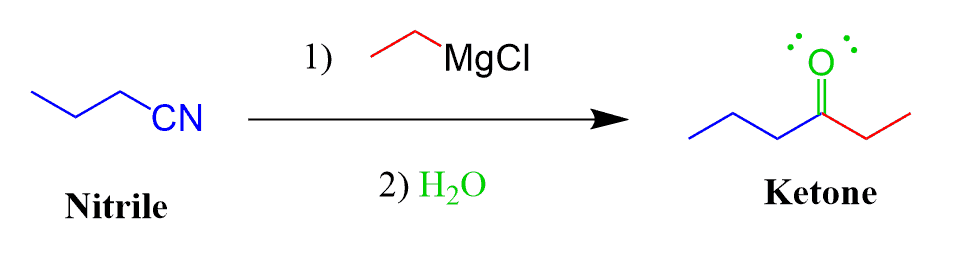
Doesn’t this look strange since we have been saying all this time that ketones react with the Grignard?
Here is how it happens.
In the first step, we have the nucleophilic attack forming the C-C bond. The product of this step is an imine in deprotonated form:
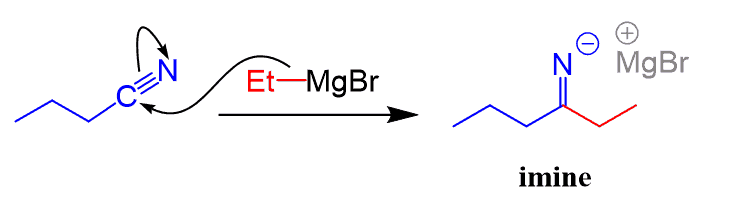
And even though the imine contains a C=N bond, it does not undergo another nucleophilic attack by the Grignard since that would put a double negative charge on the nitrogen, whichis highly unstable:
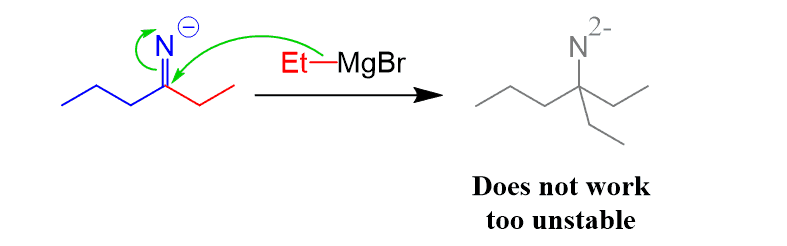
Instead, the reaction is stopped at this point by adding water or an acid since the Grignard cannot react with the negatively charged imine. The protonation of the imine also quenches the Grignard reagent:
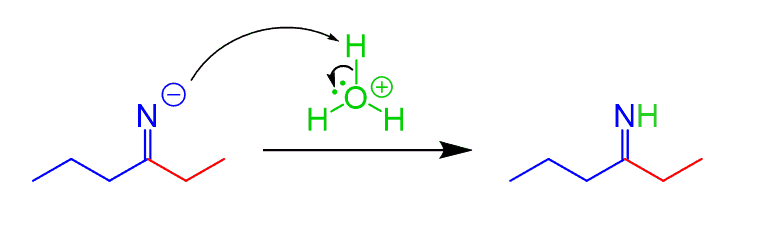
Wait, this does not show how the ketone is obtained…
You may not have covered this in your class, but imines undergo hydrolysis to form a ketone, which is the final product of the reaction. The mechanism of imine hydrolysis is shown below:
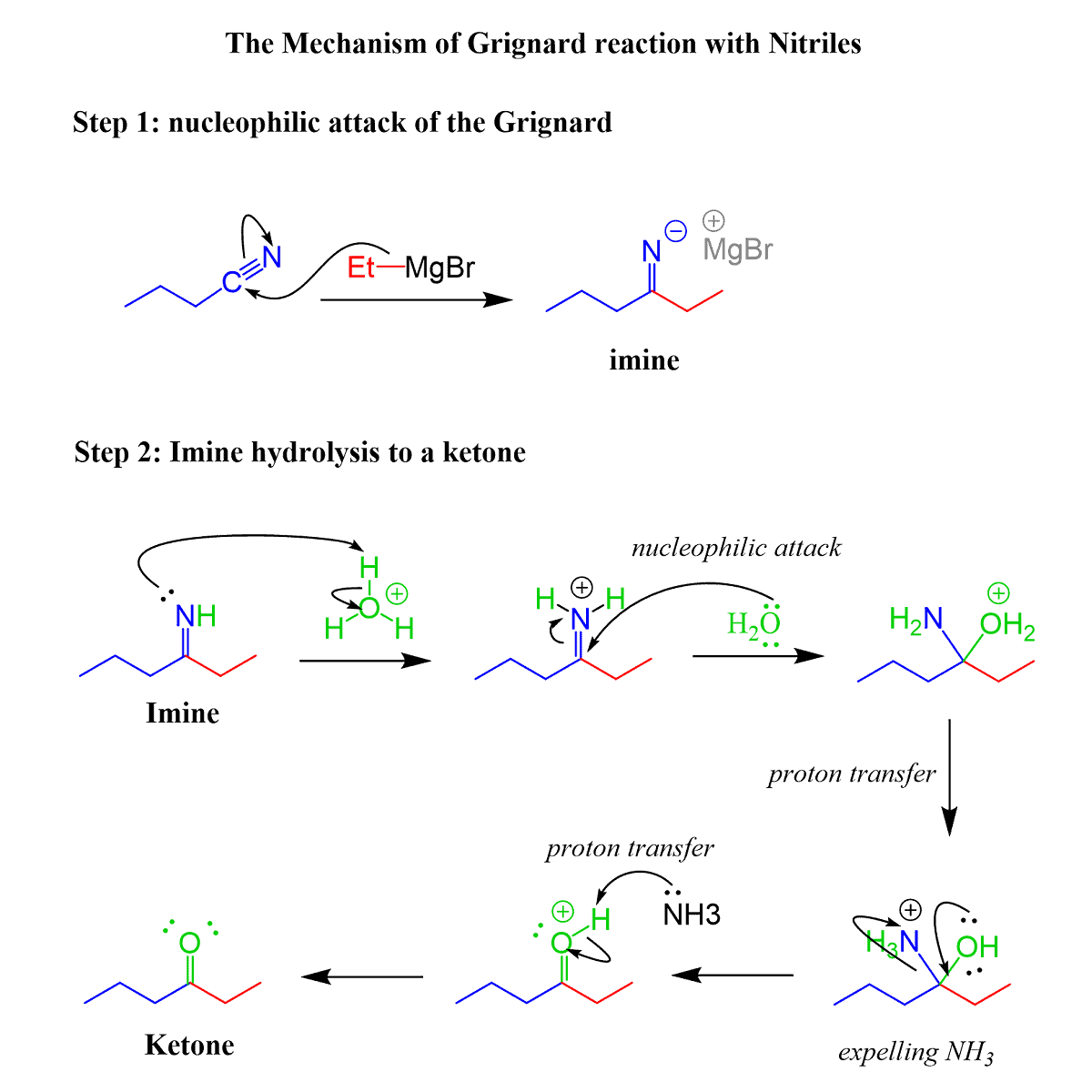
Grignard Reaction with Epoxides
Epoxides react with Grignard reagents to form alcohols:
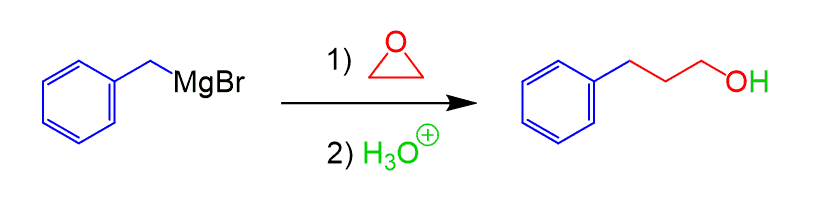
The difference with other Grignard reactions is that the epoxide does not contain a π bond, and its reactivity is a result of the ring strain. Three-membered rings are susceptible to nucleophilic attacks, as we have seen in the reactions of alkenes, such as the chlorination and oxymercuration of alkenes and alkynes. Check this post for details and examples of epoxide ring-opening reactions with additional practice problems.
With this said, the carbon atom in the epoxide is still partially positively charged, which makes it an electrophilic center for the Grignard reagent. The nucleophilic attack of the Grignard reagent opens the epoxide ring, forming an alkoxid,e which is then protonated to produce an alcohol:

Grignard reagents are good nucleophiles and the attack on unsymmetrical epoxides occurs at the less substituted carbon:
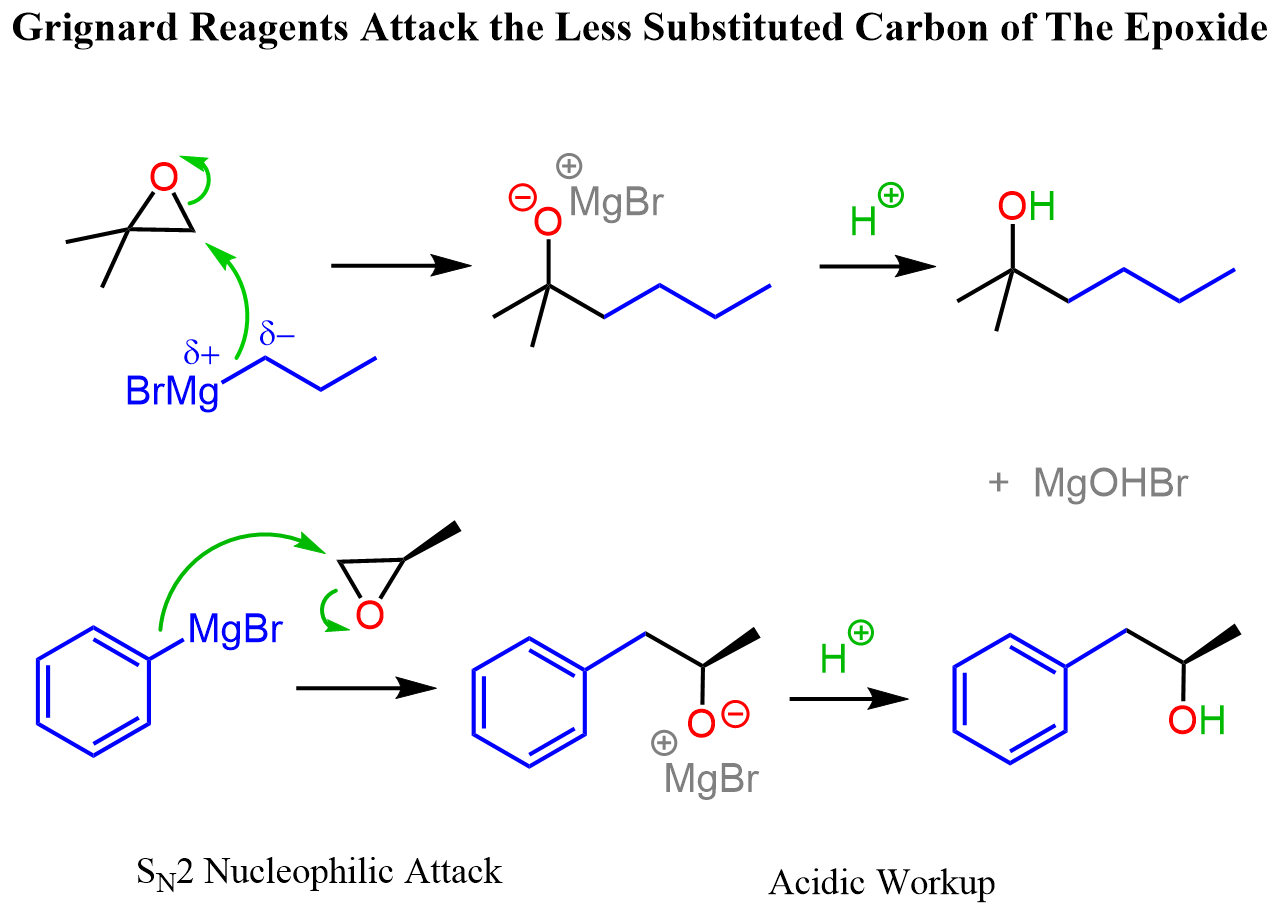
Notice that there are stereochemical considerations to the reaction as well. We have a dedicated post on the reactions of epoxides with Grignard reagents to discuss these and other features in more detail.
To summarize what we have discussed, the Grignard reaction works for compounds containing a carbonyl, such as aldehydes, ketones, esters, nitriles, carbon dioxide, and epoxides, due to the ring strain.
It is a great tool in organic synthesis, and there are a variety of ways it can be used to achieve synthetic reformations. Check the next post for more details and specific examples:
Grignard Reaction in Organic Synthesis with Practice Problems
In the following practice exercise, we will determine the major product of a Grignard Reagent with an aldehyde, ketone, ester, carbon dioxide, an ether a nitrile.


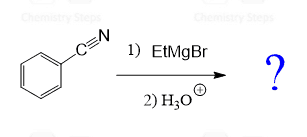
Why is ethoxide agood leaving group?
Assuming you are talking about the reaction of epoxides with Grignard reagents, it’s not that ethoxide is a good leaving group – it’s that the others, which are carbanions, are worse. Thus, the tetrahedral intermediate expels the ethoxide to restore the C=O bond and eliminate the negative charge.