Consider these E2 elimination reactions. For both, the β hydrogens are on the right side of the Br, and as expected, that’s where the double bond appears in the product:

So, no problems with the regiochemistry of these reactions. However, the stereochemistry is a different story. While the first reaction gives the E alkene as the major product, the Z alkene predominates in the second reaction. It is surprising since, remember, E alkenes are more stable because of steric strain in Z alkenes.
And the question is why and how one additional methyl group affected the stereochemical outcome of the reaction. Here is the short answer, but we’ll go over it in detail:

Stereoselectivity of E2 reactions
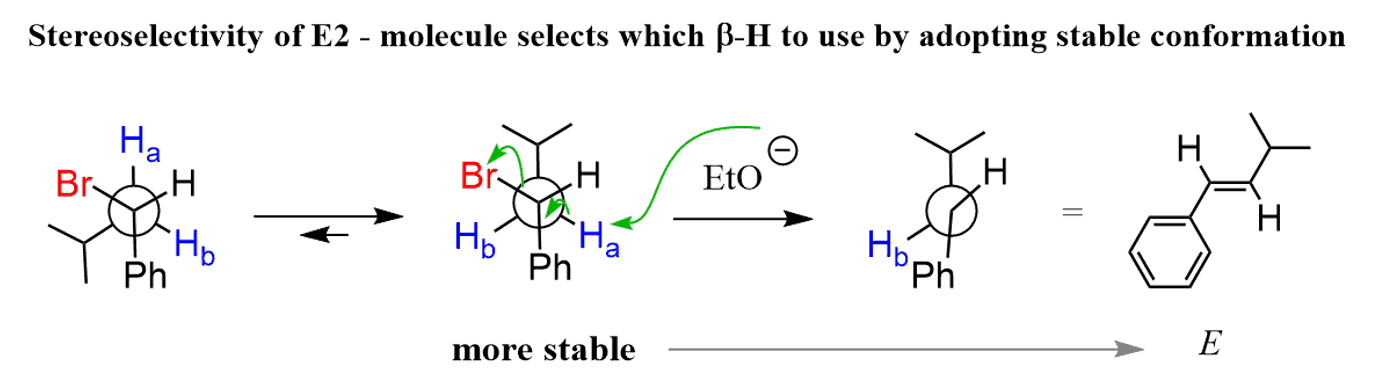
This is all explained by the geometrical requirement of the leaving group and the β hydrogen in the E2 mechanism. The transition state of the E2 mechanism requires an antiperiplanar orientation of the leaving group and the β hydrogen. Simply put, they need to be at 180o. Let’s check this by using Newman projections and sawhorse representations, whichever works for you the best. We will be looking through the C1-C2 bond, where the leaving group and the β hydrogens are:

Using the Ha was one option that gave the expected E alkene. However, if we rotate around the C1-C2 single bond, it will be possible to align the Hb at 180o with the Br as well:

So the elimination can occur from both conformations of the substrate since there are two β hydrogens. This is a stereoselective elimination – the molecule “selects” which β hydrogen to use to produce the most stable alkene (E is more stable than Z).
Stereospecificity of E2 reactions
Now, let’s draw the Newman projection of the second substrate:

In this case, there’s only one β hydrogen and therefore only one confirmation that allows us to put it at 180o with the Br. It is not the most stable conformation, but the molecule has no choice, as that is a requirement. Therefore, this is a stereospecific reaction.
So, to determine whether it is a stereoselective or stereospecific E2 reaction, check the number of beta hydrogens:

- If there are two β hydrogens, it is stereoselective.
- If there is only one β hydrogen, it is stereospecific.
It is worth mentioning that not all stereospecific E2 reactions give the Z alkene. It depends on the stereochemistry of the substrate, and as long as the β hydrogen is aligned antiperiplanar to the leaving group, the corresponding alkene will be formed (E or Z)
A shortcut to stereospecific E2 reactions
Predicting the product of a stereospecific E2 reaction by drawing Newman projections is great for explaining the concept, yet it is time-consuming. So, let me give you a quick tip.
To quickly predict the correct stereoisomer of a stereospecific E2 reaction, check the wedge and dash of the beta hydrogen and the leaving group:
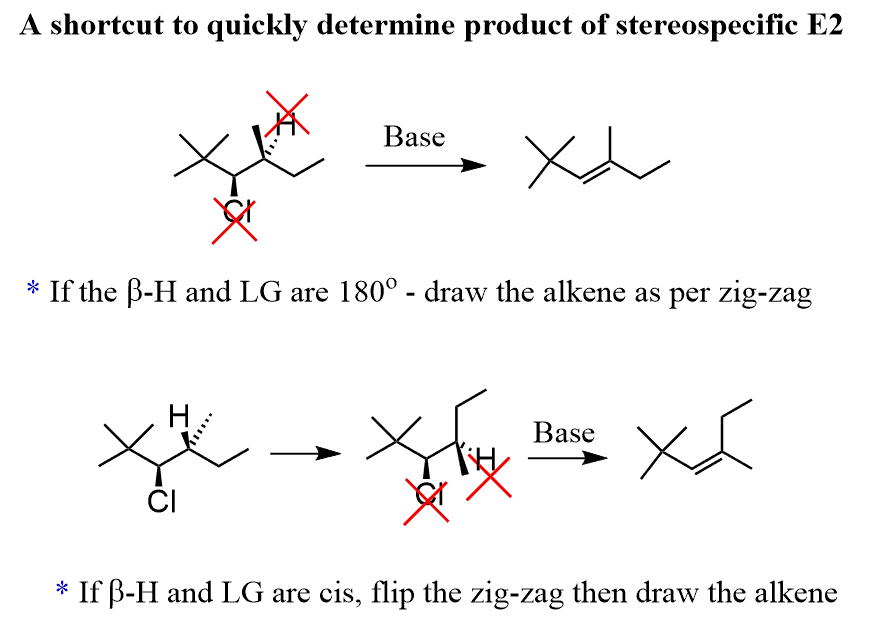
If one is a wedge and the other is a dash, then it is good to go – simply erase them and place a double bond between these two carbons in the corresponding alkene.
If there are cis (wedge-wedge or dash-dash), you need to flip the groups on the β carbon. As a result, the alkene does not have the configuration as predicted from the zig-zag structure of the substrate:


Fluent and simple love you ❤️
Amazing content.
Love it
Thank you.