Let’s state the objective of this post right away. We need to explain why the following reaction produces one of the alkenes as the major product:

This preference for addition to alkenes was first explained by Markovnikov and is known as Markovnikov’s rule which states that:
In the addition reaction of HX to an unsymmetrical alkene, the H adds to the carbon that already has the greater number of hydrogen atoms. So, visualize it as “H goes to Hs”.
We can also formulate it as such: In the addition of HX to an unsymmetrical alkene, the X atom (or group) adds to the carbon that already has the greater number of carbon atoms (more substituted carbon atom). Kind of like the rich get richer:

To understand why the first alkene is the major product, we first need to remember the stability of carbocations.
More substituted carbocations are more stable because of the electron-donating effect of alkyl groups and the hyperconjugation.

Hyperconjugation is the charge stabilization by pushing some electron density of the adjacent σ bond to the empty p orbital of the carbocation:

Now, let’s draw the mechanism of the electrophilic addition of HBr to the alkene. The first step is the Lewis Base reaction, where the π bond electrons attack the proton. And we have two options here – one is to put the H on the right carbon (C1) and the second is to put it on carbon number 2:
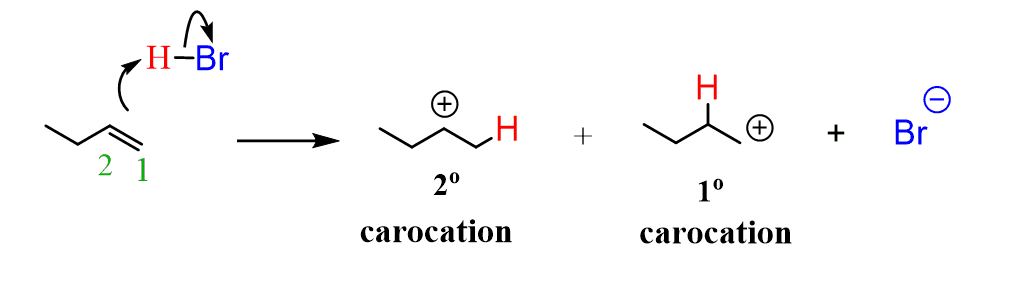
Now, what is the preferred path here (which carbocation is stable)?
The first carbocation is more stable because it is disubstituted (secondary carbocation) while the second one is monosubstituted (primary carbocation).
Therefore, there is a large excess of it in the reaction mixture, and in the second step, the nucleophilic attack of the bromide ion forms the secondary bromobutane as the major product:
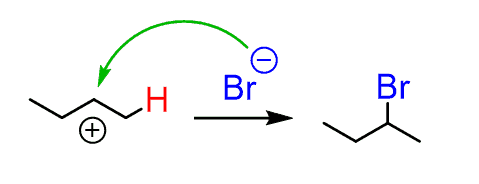
The protonation of the alkene is endergonic and requires a high energy of activation. Therefore, it is the slow, rate-determining step while the nucleophilic attack of the halide quickly stabilizes the carbocation.
Regioselectivity of Alkene Addition Reactions
You might have noticed that the two alkyl halides mentioned in the previous reaction are regioisomers (constitutional isomers). And because a reaction that produces one of the regioisomers in greater amounts is regioselective, the addition reaction of HX to alkenes belongs to this class as well. Hydrohalogenation of alkenes is a regioselective reaction.
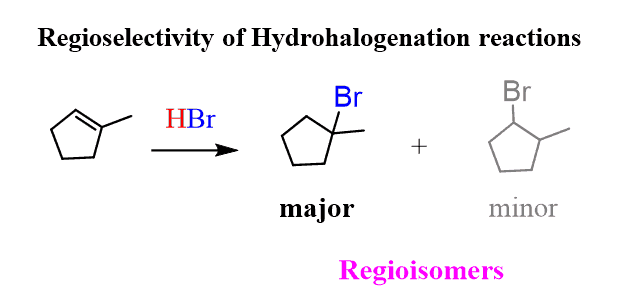
So, essentially, Markovnikov’s rule demonstrates the regioselectivity of the electrophilic addition reactions to alkenes. It is very important to understand the mechanism of the addition reactions and the concept behind Markovnikov’s rule as it lays the basis of a lot of reactions of alkenes, alkynes, and aromatic compounds.
Markovnikov and anti-Markovnikov additions
Although Markovnikov’s rule was initially described by observations from the addition of hydrogen halides to alkenes, it is also used to explain the regioselectivity of other reactions such as the acid-catalyzed hydration of alkenes. Interestingly, it was observed over time that this selectivity only works when pure reagents are used. For example, when traces of alkyl peroxides (ROOR) are present in the reaction, the regioselectivity is reversed:
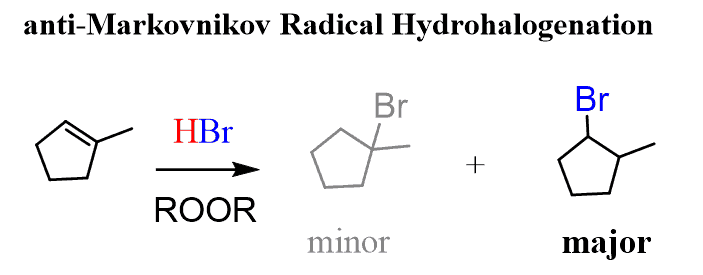
The reason for this is the change of the mechanism from ionic to a radical mechanism (radical hydrohalogenation) triggered by the peroxide.
In general, any addition reaction that goes against Markovnikov’s rule is described as anti-Markovnikov. Another good example is the Hydroboration oxidation of alkenes and alkynes.


In the solutions for Part 2, you say “produces to enantiomers”. Do you mean two enantiomers? Also, why is there no dashes/wedges for answer B in the same section?
The wedge and dash notations are most often used when there is a chirality center and since the carbon with the Br is not chiral, there is no need for wedge and dash lines. That specific molecule is achiral because of a plane of symmetry. I fixed the part about enantiomers. Thanks.
You can read more about symmetry and chirality in this article:
Symmetry and Chirality. Meso Compounds
Hi there, i am looking for some trans and cis alkene reactions. Are they posted anywhere, if yes can you please share the link here.
Thanks in advance.
Hello,
Cis and trans alkenes do not generally differ in their reactions. The stereochemistry of the product may be different for some reactions. You can check this article for some examples Cis product in an anti Addition Reaction of Alkenes and the quiz on alkene reactions.