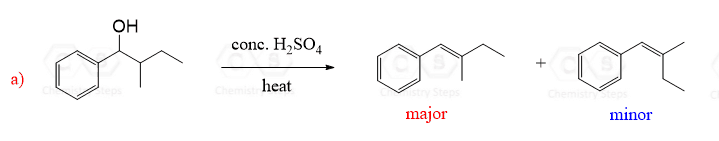
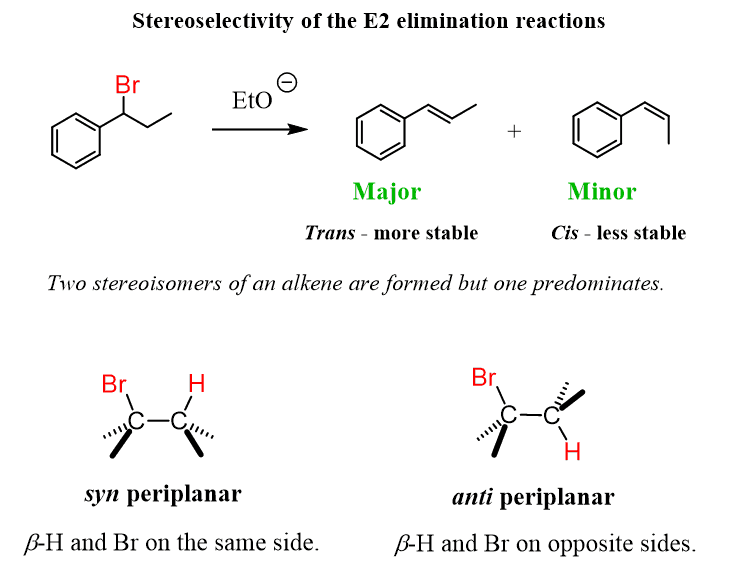
E1 reactions are stereoselective – that is, when a cis or a trans alkene can be formed, the trans isomer is generally the major product:
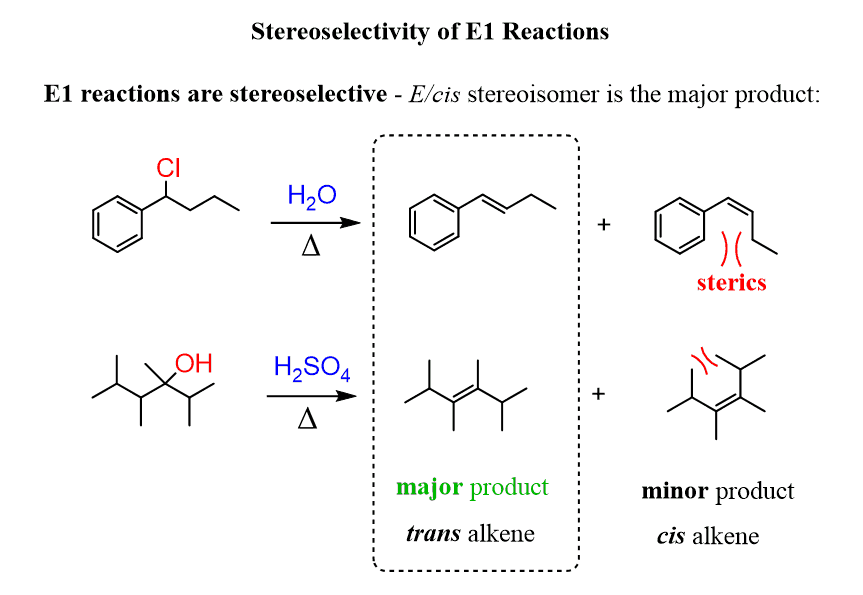
This selectivity can be explained by simply comparing the stability of alkenes. Remember, trans alkenes are more stable because of the less steric strain.
Stereochemistry of E2 and E1 Reactions
Recall that E2 reactions are also stereoselective. And there are two factors we consider here: the first is the stability of the alkenes, and the second is the required anti-periplanar geometry of the β-hydrogen and the leaving group.
Whenever possible, the cis/E alkene is the major product. However, despite the stability of trans alkenes, the anti-periplanar orientation in the E2 mechanism sometimes leads to the formation of the less stable – cis alkene dictated by the initial configuration of the α and β carbons:

This is the stereospecificity of the E2 reaction.
On the other hand, E1 reactions do not require an anti-periplanar geometry since the loss of the leaving group happens before the removal of the β-hydrogen. This allows the carbocation intermediate to adopt the more stable anti-conformation, which leads to the trans alkene as the major product:
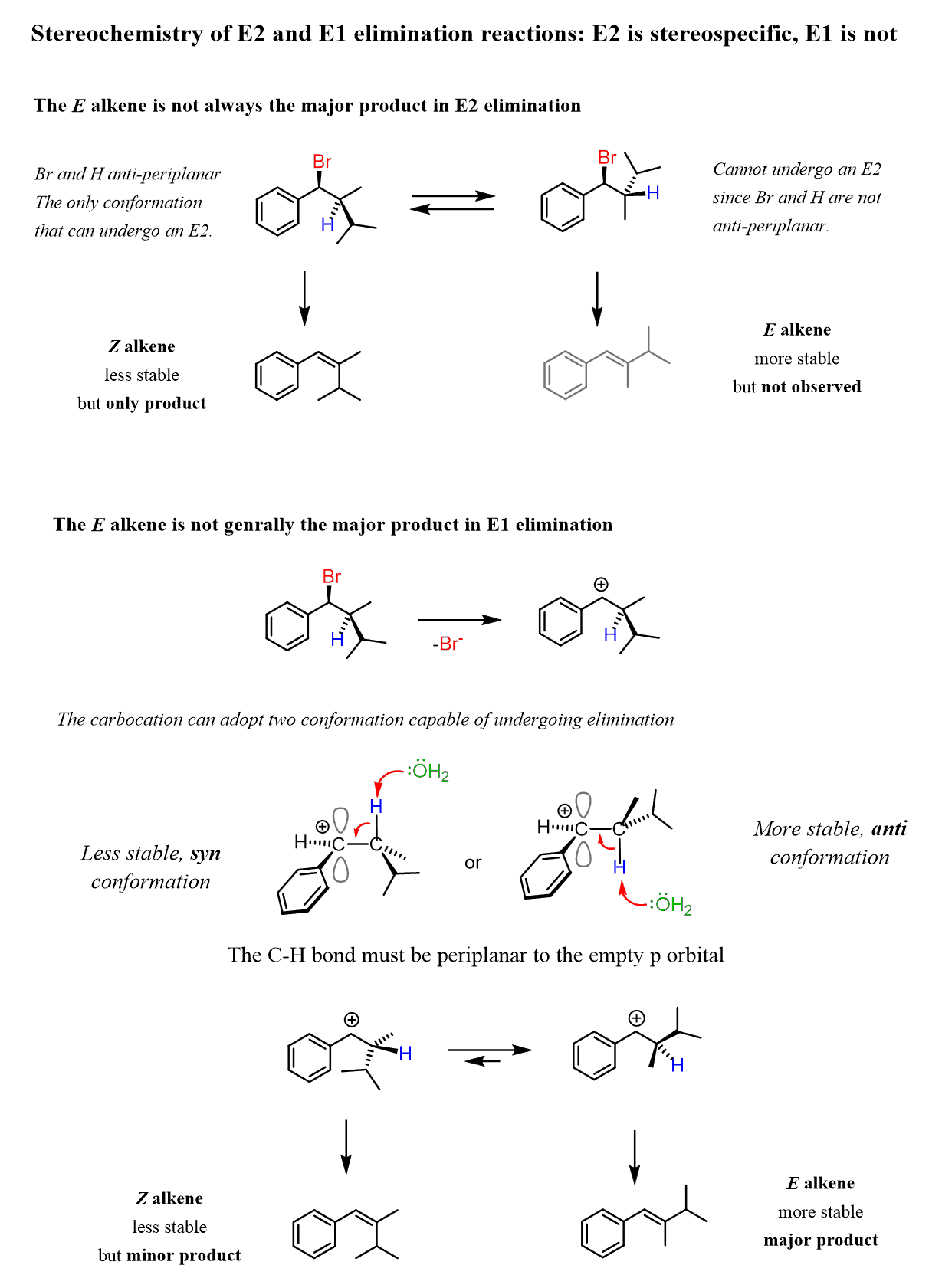
To summarize, the E1 reaction favors formation of the more stable E (trans) alkene regardless of the initial stereochemistry of the substrate. It is a stereoselective reaction.
For the E2 reaction, whenever possible, it will favor the E (trans) alkene. However, depending on the initial stereochemistry of the substrate, the Z (cis) alkene may be the only product – stereospecific elimination.
Both the stereoselectivity and stereospecificity of E2 reactions are covered in separate articles listed below: