Radicals are reactive species with a single unpaired electron. They are formed by homolytic cleavage of a covalent bond, meaning that each atom gets one electron of the covalent bond upon its breaking. An example is the hemolysis of halogens, which sets the initiation of radical halogenation:

Our focus is going to be on the stability of carbon radicals, so let’s mention that the carbon here is sp2 hybridized with trigonal planar geometry:
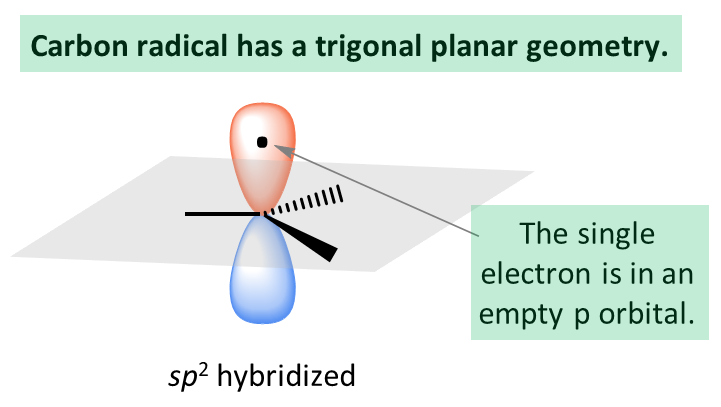
Recall that this is similar to the structure of carbocations, and they have the same pattern of stability. Remember, the stability of carbocations increases with the number of alkyl groups as these are electron-donating groups (EDG) via the inductive effect:

The radical carbon is also electron-deficient, and therefore, electron-donating groups increase its stability. We have seen earlier that alkyl groups are inductive electron donors, and as expected, tertiary radicals are more stable than secondary, which, in turn, are more stable than primary and methyl radicals:

Notice that allylic radicals are more stable than tertiary radicals because of the resonance stabilization by the neighboring double bond.
Bond Dissociation and Radical Stability
The stability of the radicals is measured based on the values of bond dissociation energies. Remember, these are the enthalpy changes associated with homolytic bond cleavage. The smaller the value of bond dissociation, the easier it is to break the bond:
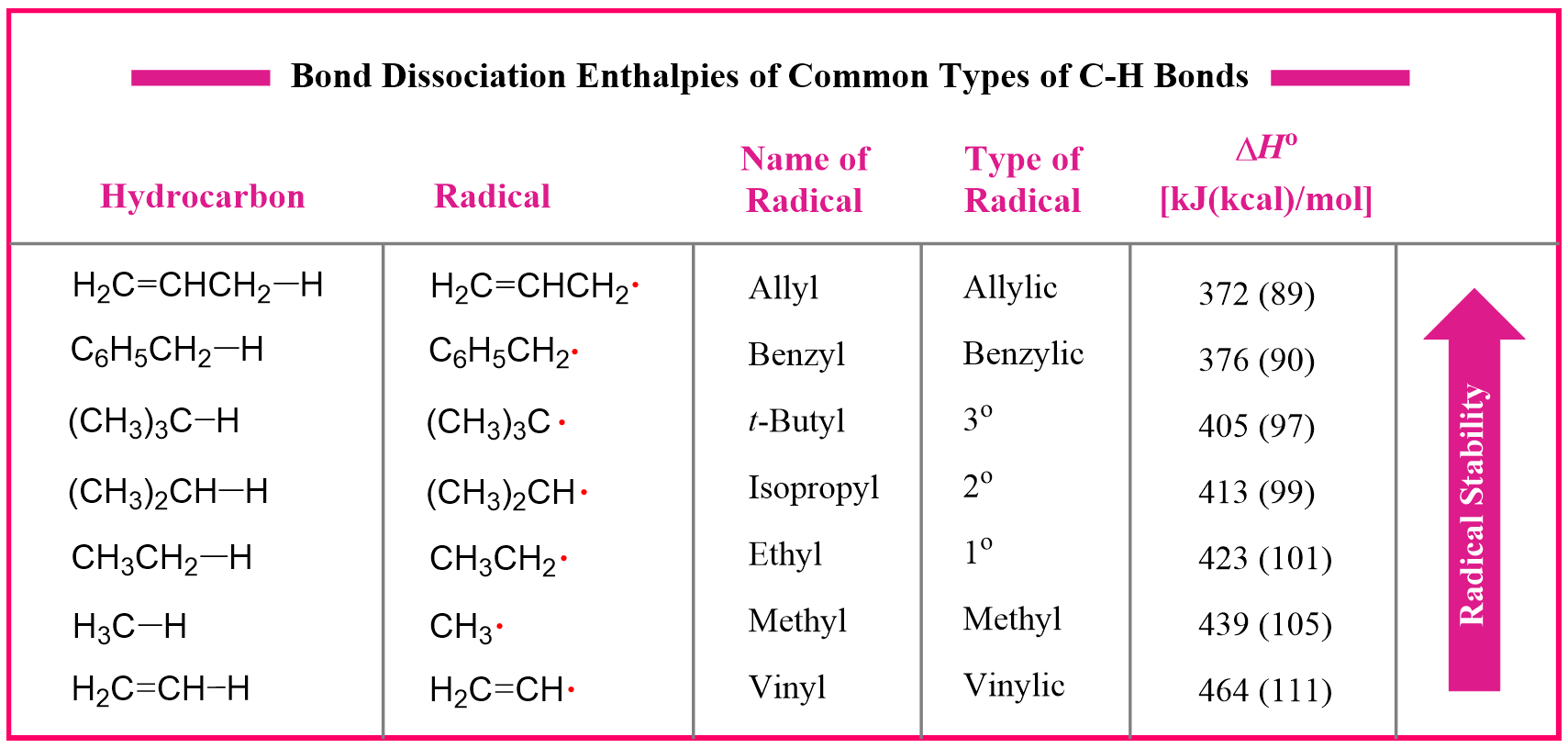
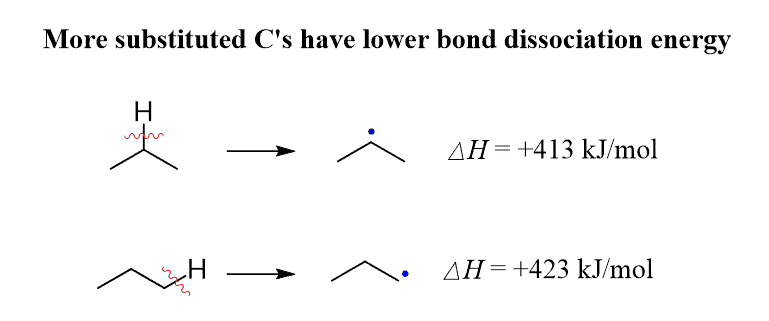
As a visual example, below are shown the values of DH ° for the primary and secondary C−H bonds of propane:
Reactions that Demonstrate the Radical Stability
The pattern for radical stability is confirmed by the selectivity of radical halogenation. For example, the bromination of propane occurs primarily on the secondary carbon because of the greater stability of the corresponding radical intermediate. The selectivity is a little lower for chlorination, and we discuss this in the article on the halogenation of simple alkanes:

Another reaction demonstrating the resonance-stabilization of radicals is the selective bromination of the allylic position. For example, but-1-ene has a primary and an allylic position, and when treated with NBS, the bromination occurs in the allylic position:

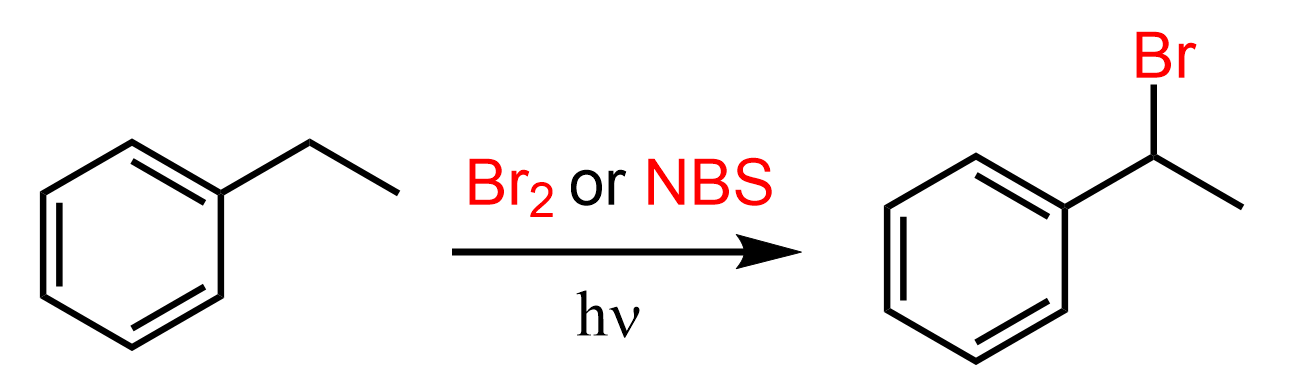
Notice that the intermediate allylic radical is resonance-stabilized by the π bond. Similar to this is the selective bromination and chlorination of the benzylic position:
Check Also
- Stability of Carbocations
- Free-Radical Addition of HBr: Anti-Markovnikov Addition
- Initiation, Propagation, and Termination in Radical Reactions
- Selectivity in Radical Halogenation
- Resonance Structures of Radicals
- Stereochemistry of Radical Halogenation with Practice Problems
- Allylic Bromination by NBS with Practice Problems
- Radical Halogenation in Organic Synthesis

This article doesn’t seem to be working. I can’t see a practice problem or reading section.
Yeah me too! There seems to be nothing here