Aldehydes and ketones have higher priority than all the other functional groups we covered so far. Therefore, they define the parent chain and give the corresponding suffix. All the other groups standing below in the functional group priority table are added as a prefix.
Here is a table of functional group priorities for the reference:

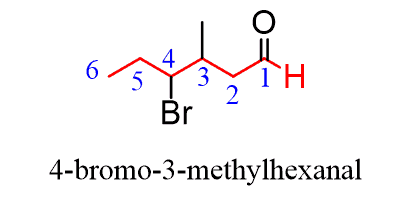
So, to name a compound containing an aldehyde and any functional group stranding below it in the table, you need to first find the longest carbon chain containing the -CHO group and change the suffix from “ane” to “al”, dropping the “e” and the locant “1” in the final name:

Everything else is based on the IUPAC nomenclature rules for simple alkanes.
The substituents are placed in alphabetical order:
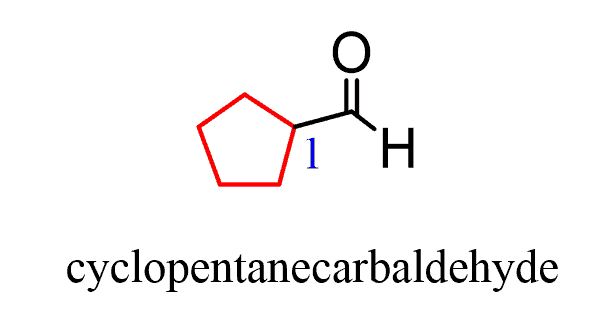
The only out-of-normal situation you may encounter is when the -CHO group is on a ring. In this case, the suffix changes to “carbaldehyde”:
If substituents are also present, the numbering starts from the aldehyde and goes in the direction that minimizes the numbering of the substituents:

Naming Ketones
Ketones have lower priority than aldehydes. However, if it happens to be that the ketone is the highest priority in the molecule, then the suffix changes to “one”.
So, to name a ketone, we need to choose the parent chain such that it is the longest carbon chain that contains the C=O group:

The locant indicating the position of the carbonyl group can be placed before the parent or before the suffix “one.” Both names are acceptable according to the IUPAC recommendations. I.e., hexan-2-one or 2-hexanone would be suitable names.
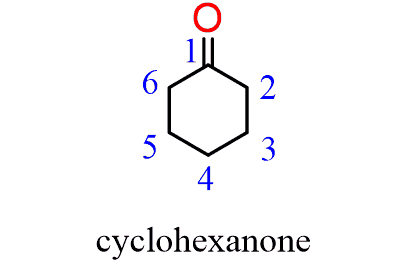
When naming a cyclic ketone, start numbering the ring beginning with the carbon connected to the C=O group. This rule always puts the =O group at C1, therefore, the “1” is usually omitted from the name:
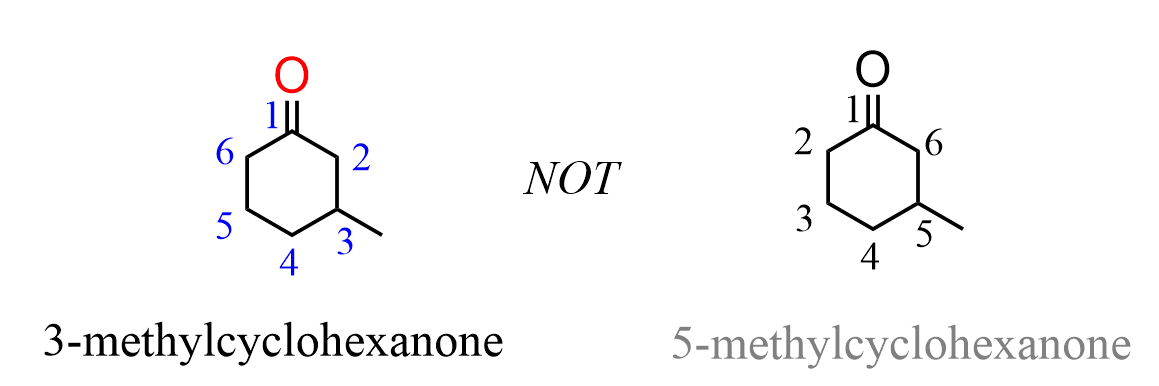
When other groups are present on the ring, it is numbered clockwise or counterclockwise depending on which direction gives the next substituent the lower number:
Any stereochemistry, such as the R and S and E and Z configuration of a double is addressed regardless of the priority in the molecule:
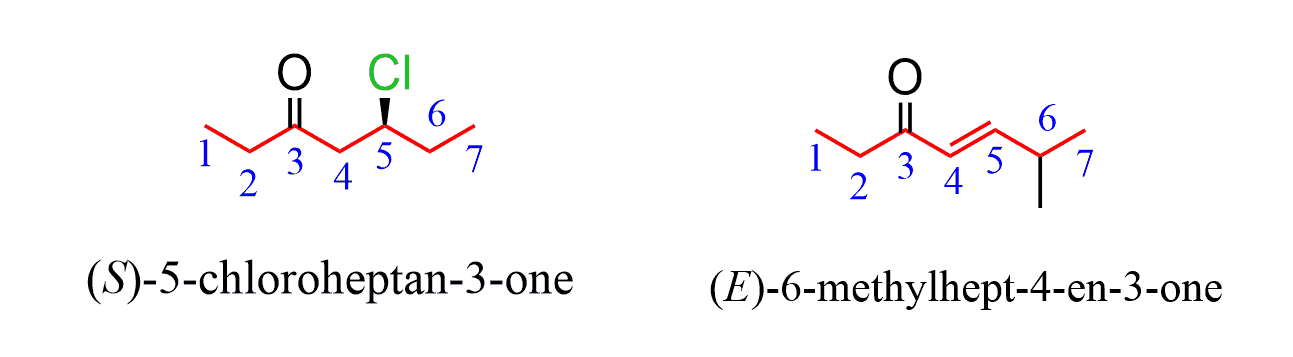
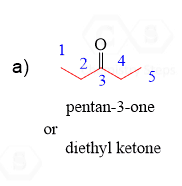
Aldehydes and Ketones can also be identified by their common names:




Good
Hye doctor just to note that with the common names of the ketons you also put examples of the aldehydes which might be a little confusing to clearly differentiate between the two groups. Personally, i dont have a problem with it however some might find it it confusing and harder to study.
Hi there, thanks for the note. I have changed it to “Aldehydes and Ketones”.