Importance of radical Halogenation
The source of the large majority of organic materials used for a countless number of applications, which can be plastics, drugs, paints, clothes, electronics and many more is the crude oil.
We need to have a hydrocarbon chain to start building on it. A great part of the crude oil is the alkanes which, based on the hydrocarbon chain length, make the natural gas, gasoline, diesel, asphalt and etc.
All of that is important, however, has nothing to do with the beauty of organic chemistry which gives the ability to design and create new molecules with sophisticated geometries and properties.
The reason for this limitation is that, if you paid attention, in all the chapters where you did some synthesis, you always needed a functional group. The functional group(s) is what brings the unique properties and reactivity of organic molecules. There is not much you can do from, for example, propane except for burning it like fossil fuel.
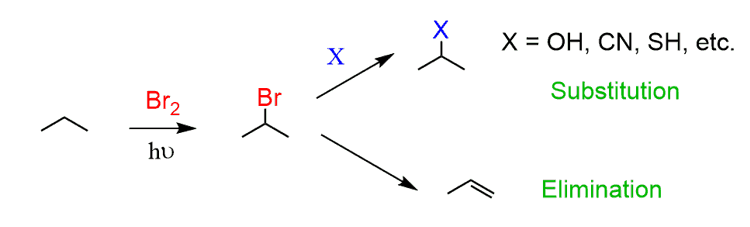
One of the few, chemistry-wise meaningful things to do with alkanes is reacting them with halogens. This reaction produces alkyl halides which can be used in many reactions. The simplest would the substitution and elimination reactions.
For example, the bromination of propane will selectively produce the 2-bromopropane which can then be used as an electrophile in substitution and elimination reactions:
Organic Synthesis and Radical Halogenation
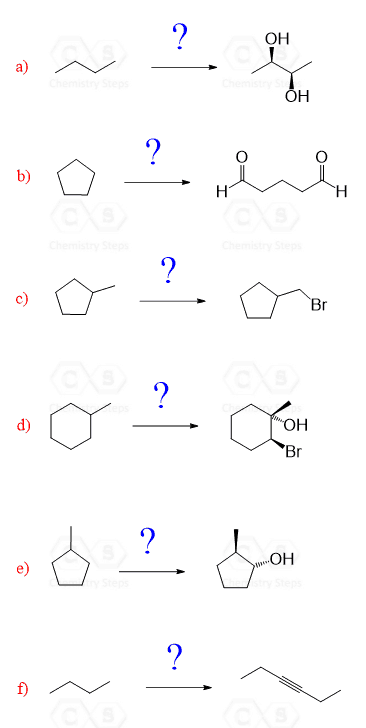
Let’s see how the following molecules can be synthesized from 3-methylpentane.
Try to solve the problems before checking the solutions and as a hint, remember that you need to start from a halogenation of the starting alkane.
Show how each molecule can be synthesized from cyclohexane:
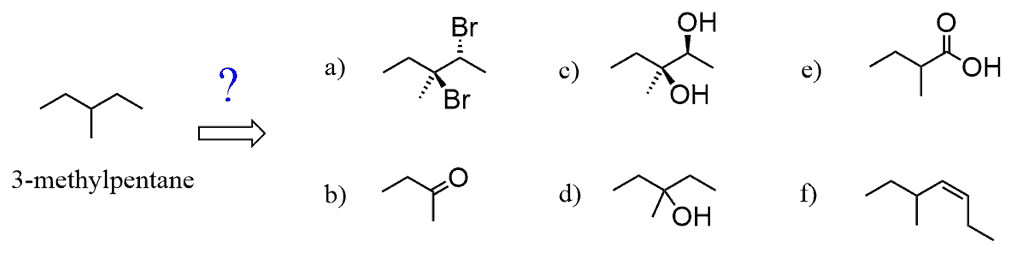
a) Seeing two halogens in anti orientation indicates that this is a product of alkene halogenation. Putting this in a retrosynthetic scheme, we can see that the precursor to the anti-dibromo substrate is the 3-methylpent-2-ene:
Therefore, we need to think about how to prepare this alkene from the starting alkane.

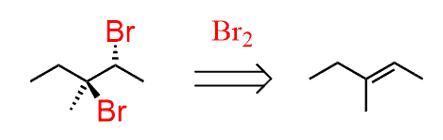
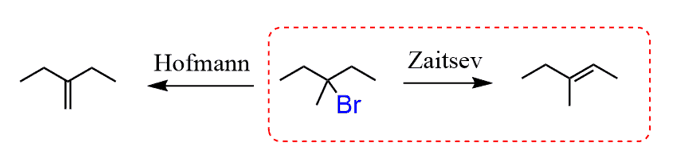
The first step has to be a halogenation and we need to do a bromination since it is the only regioselective halogenation. Remember, the Br goes to the more substituted carbon:
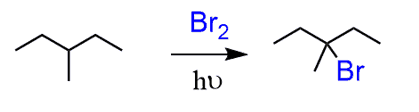
Treating the 3-bromo-3-methylpentane with a strong base will result in an E2 reaction. However, you should pay attention to the regiochemistry and choose between the Zaitsev and Hofmann elimination.
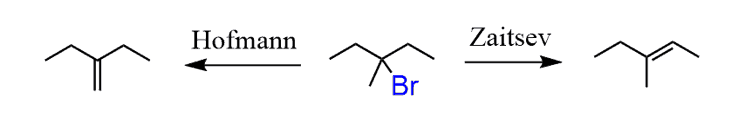
In this case, we need the more substituted alkene, so a non-hindered strong base is needed to promote the Zaitsev product:
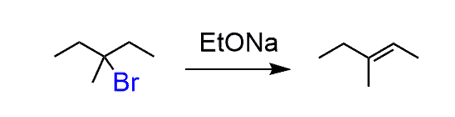
In the last step, you need to do a bromination of the alkene. Remember, the halogenation of alkenes goes through the formation of halonium ion followed by the nucleophilic attack from the opposite side of the halogen:
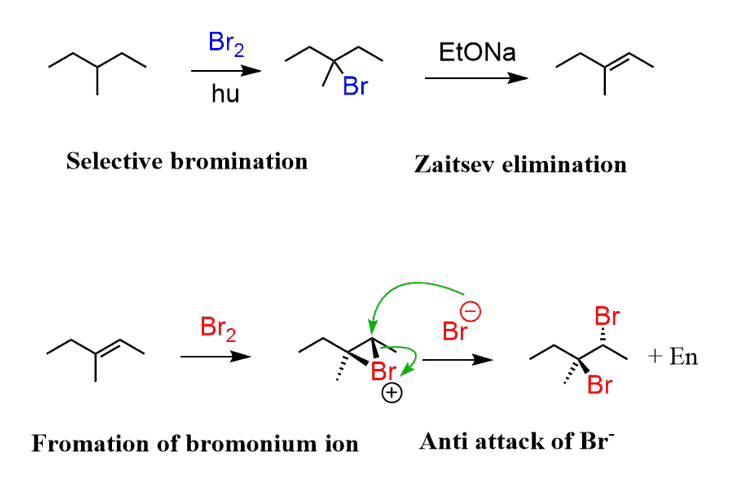
The final product is obtained as a mixture of two enantiomers since the bromination is not stereoselective.
b) Looking at the starting material and the target product, it is clear that the number of carbons has changed. It is always a good idea to number the carbons so that you can keep track of their number. In this case, we started with five carbons in the main chain and ended up with four in the product. And only three are part of the original main chain:
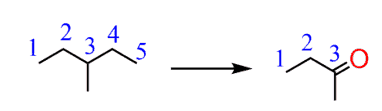
Another thing to pay attention here is the ketone functional group in the product.
To decrease the number of carbon atoms, we need to cleave a C-C bond. What oxidative cleavage reaction have you learned so far? Do you see how ozonolysis could be used here?
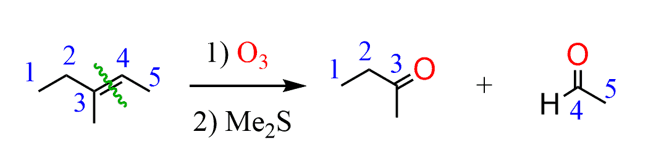
Remember the shortcut in ozonolysis of alkenes, you need to replace the C=C bond with a carbonyl.
The alkene can be synthesized as we discussed in the previous problem.
c) Cis hydroxyl groups next to each other is a hint that this must have come from a double bond again:
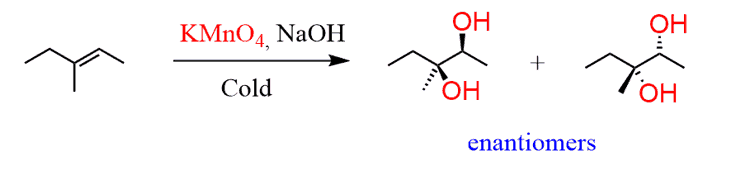
We already know how to synthesize the cyclohexene and you need to remember that alkenes allow syn and anti dihydroxylation. The cis dihydroxylation is achieved using either a cold solution of KMnO4 or OsO4.
d) In this product, you need to recognize the heteroatom (any atom except C and H is a heteroatom). You have an oxygen-carbon bond which must have come from a substitution reaction:
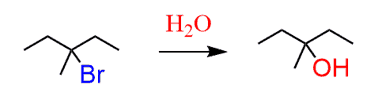
The good leaving group bromide allows performing a substitution reaction which must be an SN1 reaction since we have a tertiary substrate.
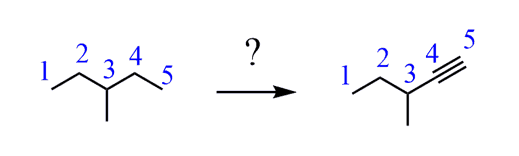
e) If you have already made a habit of numbering carbons, that is great because you have probably noticed that the product has one carbon less than the starting material.
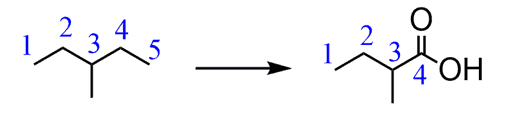
This means there was C-C bond cleavage. What C-C cleavage reactions do you know?
The ozonolysis is what most likely comes to your mind. And here you need to remember that ozonolysis of alkenes and alkynes gives different products. Namely, alkenes produce aldehydes or ketones, while alkynes produce carboxylic acid and carbon dioxide for a terminal alkyne.
Therefore, the product here is a result of the ozonolysis of a triple bond:
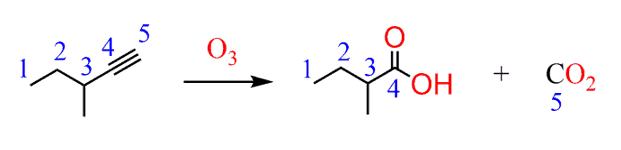
Our aim is now to convert the starting alkane to the alkyne. What do you suggest?
Recall the preparation of alkynes. You need an alkyl dihalide to react with a strong base such as the sodium amide:
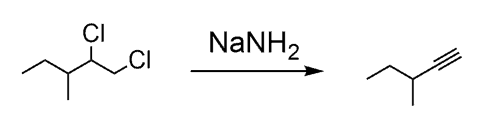
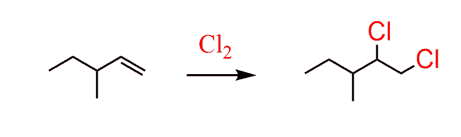
This alkyl dihalide would, in turn, come from the corresponding alkene:
And at this point, the question is how do you go from 3-bromo-3-methylpentane to the terminal alkene?
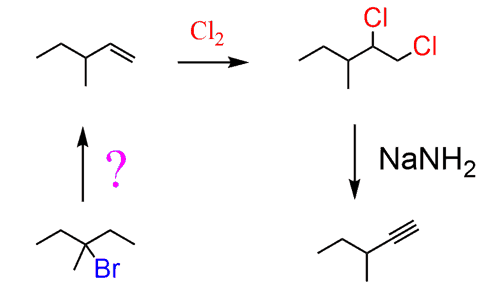
To choose correctly which alkene you need, go over changing the position of a double bond and leaving group posts. You need to move the double bond in the desired direction, which means the Zaitsev product is needed here:
Next, anti Markovnikov hydrobromination followed by a Hofmann elimination gives the desired alkene:
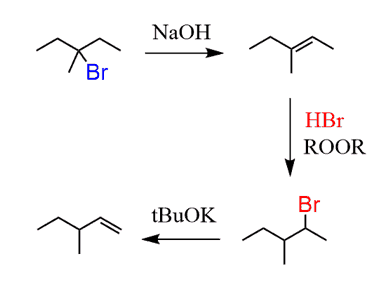
The conversion of the alkene to the corresponding terminal alkyne can be done by chlorination or bromination followed by elimination with NaNH2 as we already discussed above.
Overall, this is the synthetic scheme:
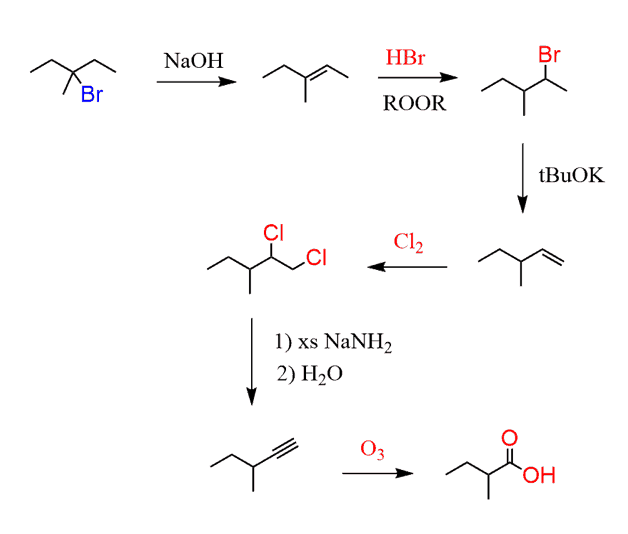
f) Comparing the product and starting material, it is evident that the carbon chain had been extended. And if you have numbered the carbon chain, you know that it has increased by two carbons.
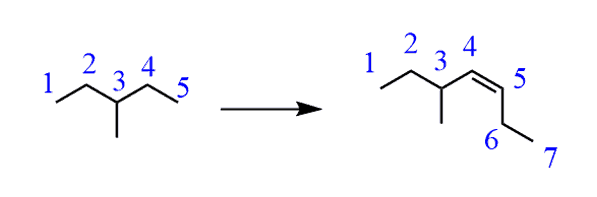
How do you extend the carbon chain organic chemistry? Well, there are many ways of doing that but; if you have only covered alkenes and alkynes in your organic class, you should always think about alkylation of terminal alkynes whenever the hydrocarbon chain is extended.
Do you see how you could apply the alkyne from the previous question here?
What about reacting with the ethyl group bearing a good leaving group?
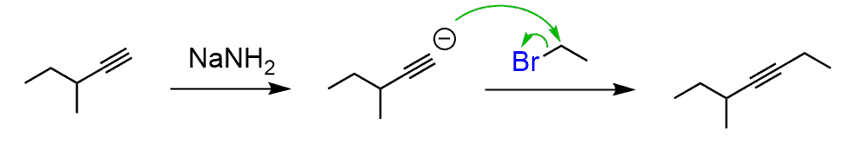
This is an example of SN2 substitution reaction and will form our target internal alkyne.
Let’s now compare the structures of the alkyne and the final product alkene. So, obviously we need to do a reduction of the alkyne and you should also notice that it is a cis (or Z in our case) alkene which requires a special reducing agent.
What reagent reduces an alkyne to the corresponding cis alkene?
If you remember the Lindlar’s reagent, great job!
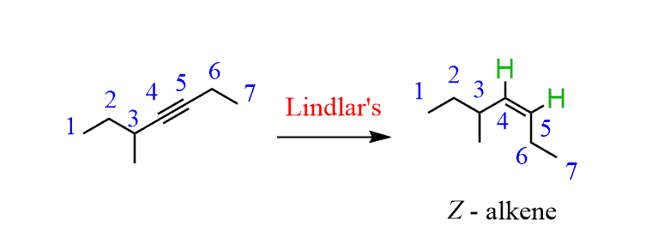
And this summarizes the importance of radical halogenation and some of the strategies used in organic chemistry that I wanted to cover today.