This is a multiple-choice quiz to practice the Naming and Reactions of Alkynes.
It comes with an 80-min video showing a detailed step-by-step solution to all the questions. It’s a must-watch, master course video!
In addition to the reactions of alkynes, it is also built on knowledge from previous chapters such as substitution, elimination, and addition reactions of alkenes.
Introduction to Alkynes
Naming Alkynes by IUPAC Nomenclature Rules
Preparation of Alkynes by Elimination Reactions
Hydrohalogenation of Alkynes
Acid-Catalyzed Hydration of Alkynes
Reduction of Alkynes
Halogenation of Alkynes
Hydroboration-Oxidation of Alkynes
Ozonolysis of Alkynes
Alkylation of Terminal Alkynes in Organic Synthesis
Alkyne reactions summary
Alkyne Synthesis Reactions
These are some example questions:
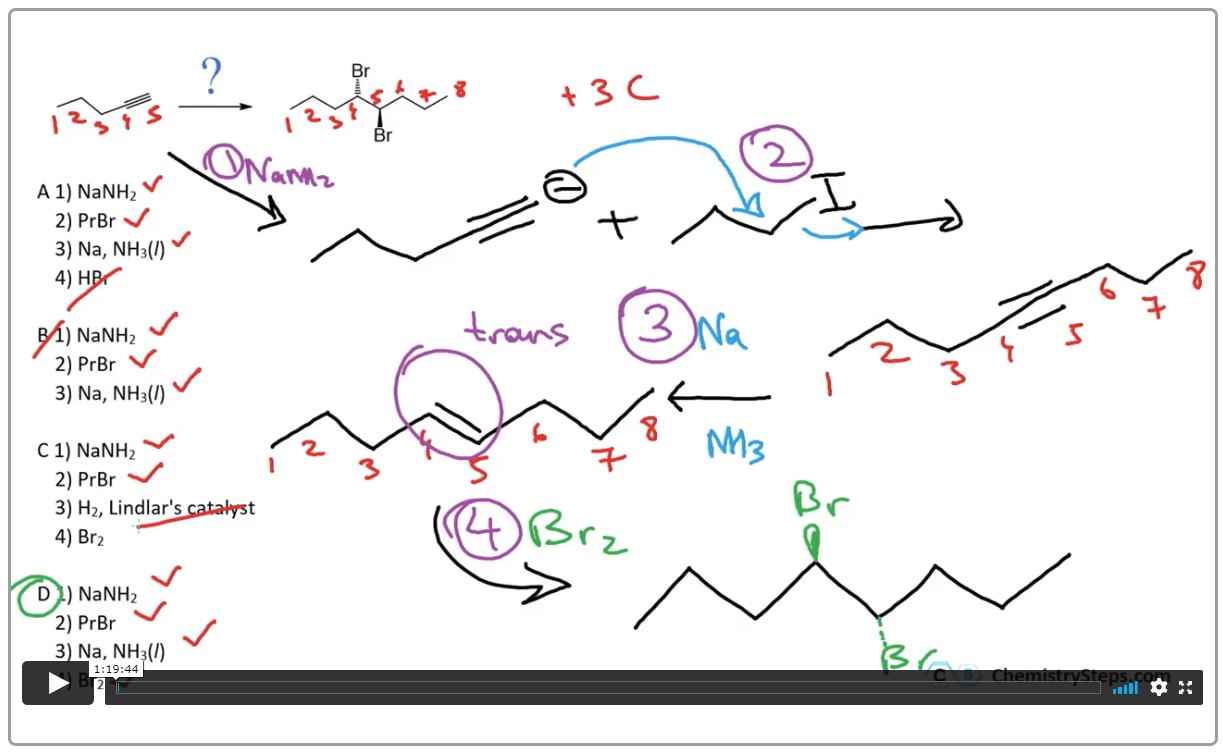
Suggest a plausible synthesis for the following transformation:

Suggest a plausible synthesis for the following transformation:


Doesn’t the Lindlar’s catalyst make cis double bonds and chlorination anti addition?
Yes, what you said is correct. Is it related to a specific question?
Sorry, it was 49. Shouldn’t chlorines be anti? I started from the end but it is not the only one like that.
Ok. So, the stereochemistry of the product depends on the stereochemistry of the starting material. If the alkene is trans, then the anti addition produces a trans product. If it is cis, the syn product is formed. It is still an anti addition but because the carbon chain of the product is drawn in a zig-zag, the relative orientation of the Cl’s is cis.
Check out this post:
Cis product in an anti Addition Reaction of Alkenes