In the previous post, we talked about the lack of reactivity of benzene towards bromination and the Kekulé structure that was suggested to explain these unique features.
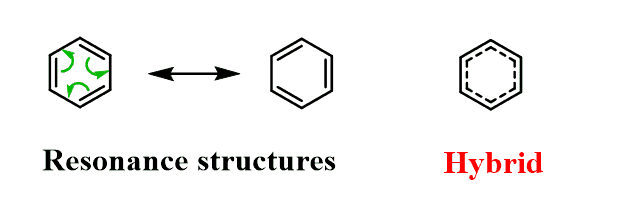
In short, Kekulé suggested that benzene is an equilibrium mixture of two compounds with alternating double bonds:

Kekulé structure satisfies the characteristics of benzene except for one, and that is the bond lengths in the ring.
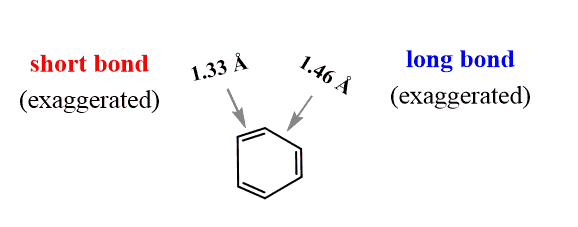
Let’s elaborate on this. It is now known that all the bonds in benzene are identical – 1.395 Å. However, if benzene existed in two resonance forms with alternating double bonds, we’d have two types of bonds: sp2–sp2 single bonds (1.46 Å) and double bonds (1.33 Å).
This would result in a distorted structure:
The perfectly symmetrical structure of benzene, however, indicates that it exists as a resonance hybrid:
The actual bond length (1.395 Å) is intermediate between the sp2–sp2 single bonds (1.46 Å) and double bonds (1.33 Å).
The common practice of using only one of the Lewis structures is only to make keeping track of the π electrons easy.
The Structure and Geometry of Benzene
All the carbon atoms in benzene are sp2 hybridized connected by sp2–sp2 single bonds and each has a p orbital perpendicular to the plane of the atoms. These p orbitals overlap, delocalizing the six electrons and making benzene a fully conjugated system. The geometry of each carbon is trigonal planar:

Let’s summarize what we know about the structure of benzene so far:
- It is cyclic
- It is planar
- All the bonds are equal
- It is fully conjugated
So, how does all of this make it very stable?
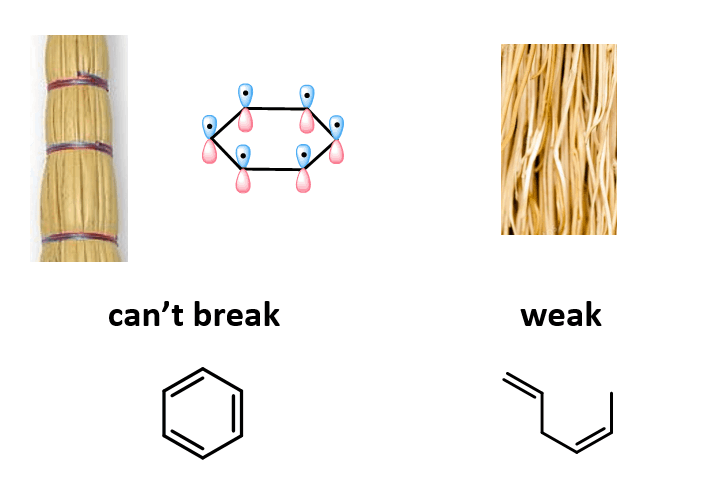
I like to think about it as a broom: you can easily break individual straws or a bunch of them when they are randomly stacked, but it wouldbe very difficult when they are all nicely aligned and tight together, just like the p orbitals of the aromatic ring:
It turned out that benzene is not the only example demonstrating the stability of the hexagon in nature. When I once visited the Temple of Garni built in the first century AD in Armenia, we went for a hike in this beautiful place known as the “Symphony of the Stones” which is a gorge where the basalt cliff walls, stretching hundreds of feet, are shaped into long hexagonal columns:

Look at all these stone-stable benzene rings!

Aromatic Resonance Stabilization
Aside from the lack of reactivity toward electrophilic addition reactions, the additional stability of benzene can also be demonstrated by the heats of hydrogenation.
Let’s compare the heats of hydrogenation for cyclohexene, cyclohexa-1,3-diene, and benzene:

These contain one, two, and three double bonds, and as expected, their stability should decrease three times going from cyclohexene to benzene and two times going from cyclohexene to cyclohexa-1,3-diene. Therefore, the heats of hydrogenation should increase as well.
Interestingly, when comparing the ΔHo values, we see that the observed values for cyclohexa-1,3-diene and benzene are lower than the mathematical predictions.
The ΔHo for the addition of one mole of H2 is -120 kJ/mol. Therefore, the expected value for 1,3-cyclohexadiene is 2 x (-120) = -240 kJ/mol. However, it is determined to be 232 kJ/mol, which is slightly smaller. This is explained by the conjugated nature of the double bonds – dienes are more stable than two isolated C=C double bonds:
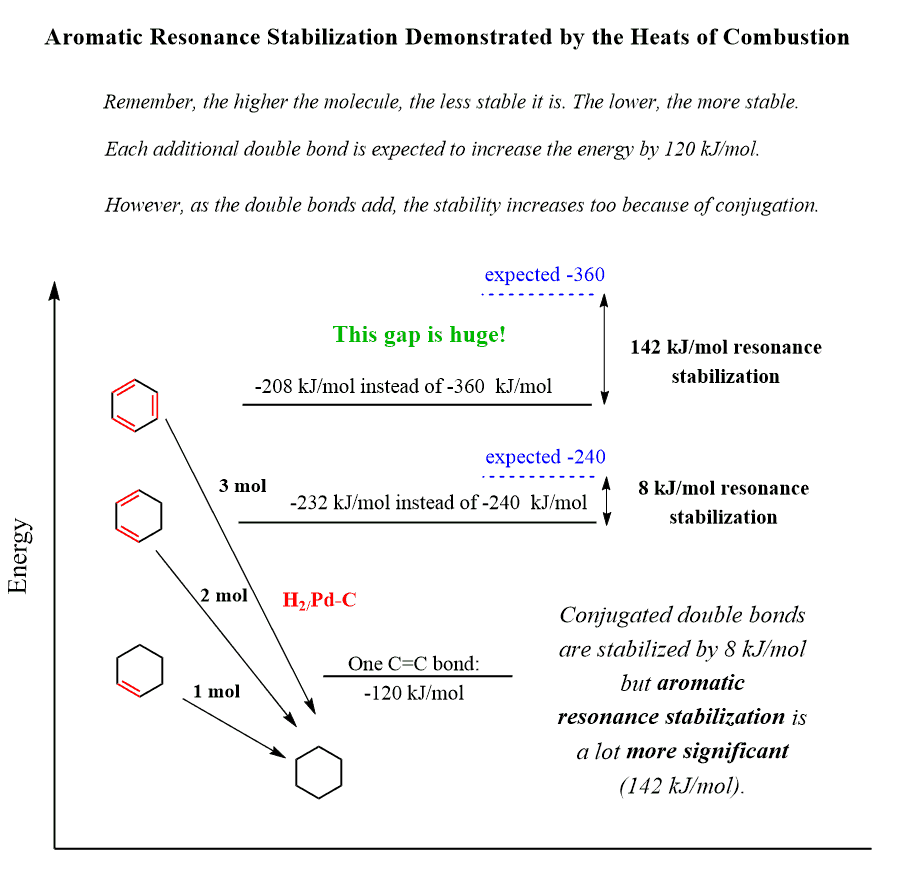
The hydrogenation of benzene to cyclohexane takes three moles of H2 and should release 3 x (–120) = –360 kJ/mol of energy if the double bonds were not related. But since 1,3-cyclohexadiene shows the additional stability of conjugated double bonds, we can expect it to be slightly lower.
However, it is not just slightly lower – the observed heat of hydrogenation is only –208 kJ/mol, which is 152 kJ/mol less than the predicted –360 kJ/mol value! Remember again that 152 kJ/mol less means it is 152 kJ/mol more stable than the calculated mathematical value.
This huge energy difference is called the empirical resonance energy of benzene – the special stability of aromatic compounds originating from the resonance and conjugation.
This would have been a good place to close the discussion about the structure and stability of benzene.
However! There is more to think about since the combination of cyclic, planar, fully conjugated, and resonance-stabilized factors does not explain why cyclobutadiene is a very unstable molecule:
What is going on here? The answer will be given when we go over the Hückel’s rule.
Check Also:
- Naming Aromatic Compounds
- Introduction to Aromatic Compounds
- Benzene – Aromatic Structure and Stability
Aromaticity and Huckel’s Rule - The 4n+2 Rule
- Identify Aromatic, Antiaromatic, or Nonaromatic Compounds
- Frost Circle
- Annulenes
- Electrophilic Aromatic Substitution – The Mechanism
- The Halogenation of Benzene
- The Nitration of Benzene
- The Sulfonation of Benzene
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- Vilsmeier-Haack Reaction
- The Alkylation of Benzene by Acylation-Reduction
- Ortho, Para, Meta in EAS with Practice Problems
- Ortho, Para, and Meta in Disubstituted Benzenes
- Why Are Halogens Ortho-, Para- Directors yet Deactivators?
- Is Phenyl an Ortho/Para or Meta Director?
- Limitations of Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Arenediazonium Salts in Electrophilic Aromatic Substitution
- Reactions at the Benzylic Position
- Benzylic Bromination
- Nucleophilic Aromatic Substitution
- Nucleophilic Aromatic Substitution Practice Problems
- Reactions of Phenols
- Reactions of Aniline
- Meta Substitution on Activated Aromatic Ring
- Electrophilic Aromatic Substitution Practice Problems
- Aromatic Compounds Quiz
- Reactions Map of Aromatic Compounds

many thanks for the information about benzene related aromaticity
very good information about benzene.
Thanks so much,I am delighted to have added something in my career of organic chemistry.