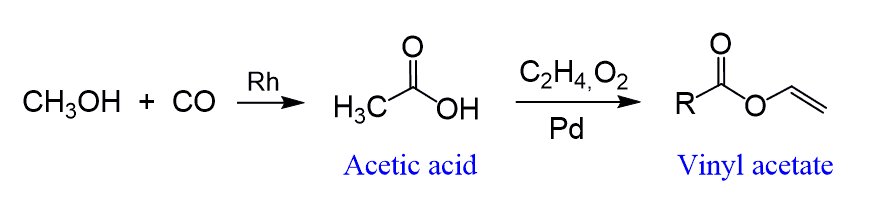
One of the most common carboxylic acids is the acetic acid which has a wide range of application starting from vinegar and going all the way to the synthesis of organic materials including vinyl acetate, which is used as a polymer (polyvinyl acetate) in paints and adhesives:

Let’s first mention one industrial method which is used to produce almost 5 million tons of acetic each year worldwide. This is achieved by metal-catalyzed carbonylation of methanol using carbon monoxide:
Most of the methods for preparing carboxylic acids in laboratory are reactions which we covered in previous topics. Therefore, they will only be shown briefly here and if you need more details or the mechanisms, follow the provided links.
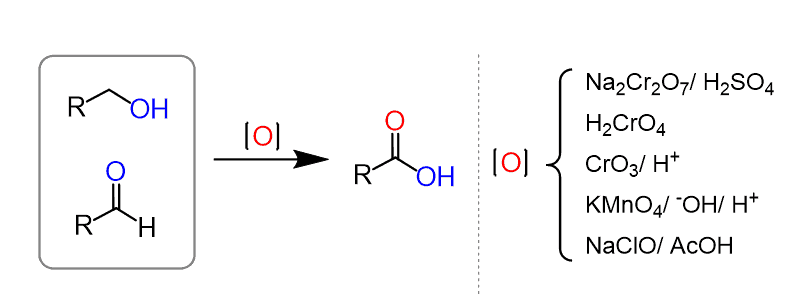
Oxidation of Alcohols and Aldehydes
One approach is to oxidize primary alcohols and aldehydes using strong oxidizing gents such as chromic acid (the Jone’s oxidation), potassium permanganate (KMnO4) or sodium hypochlorite (NaClO – bleach):
Benzoic acid can be prepared by benzylic oxidation of primary and secondary alkylbenzenes. Just like it is shown above, most strong oxidizing agents would work for this transformation:

Remember, some reagents such as pyridinium chlorochromate (PCC), pyridinium dichromate (PDC), the Swern and Dess-Martin oxidation allow for selective oxidation of primary alcohols to aldehydes. The mechanistic details and selectivity of each oxidizing agent is covered in the Oxidation of Alcohols.
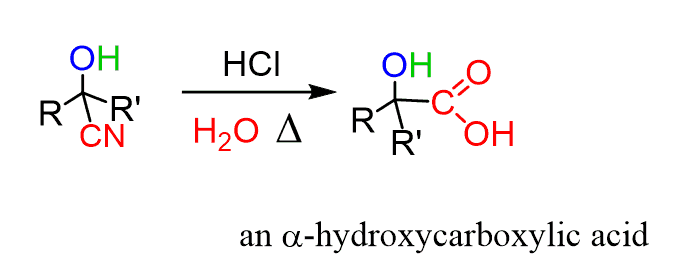
Carboxylic Acids from Nitriles
Another way of preparing carboxylic acids from alcohols and ketones is converting those into cyanohydrins followed by hydrolysis to produce ɑ-hydroxy acids:

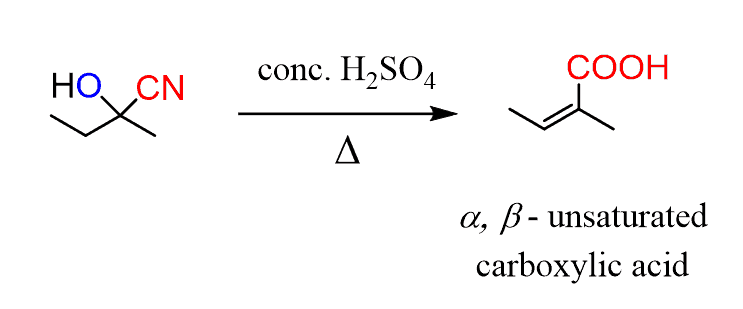
A concentrated sulfuric acid can be used to convert the resulted cyanohydrin into a α, β-unsaturated acids. Two things are happening here, first is the hydrolysis of the cyano group and second, a dehydration of the alcohol:
Of course, the conversion of the nitrile group to a carboxylic acid is not restricted to the cyanohydrins only. Any nitrile can be converted into carboxylic acids by acid- or base-catalyzed hydrolysis if no other functional groups of the molecule make it acid or base labile:
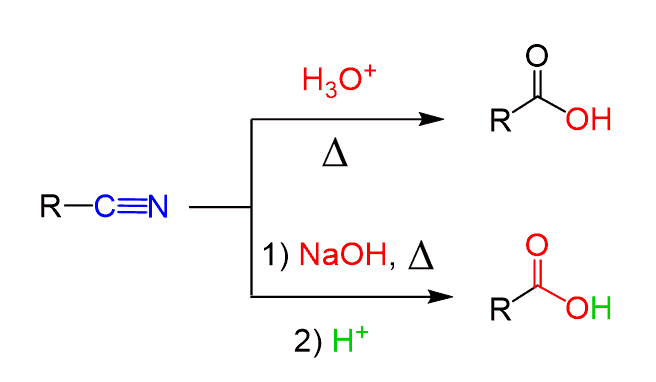
In general, introducing a cyano group is a useful strategy for synthetic transformations. For example, we can use to convert an alkyl halide to a carboxylic acid by doing first a substitution reaction followed by hydrolysis:
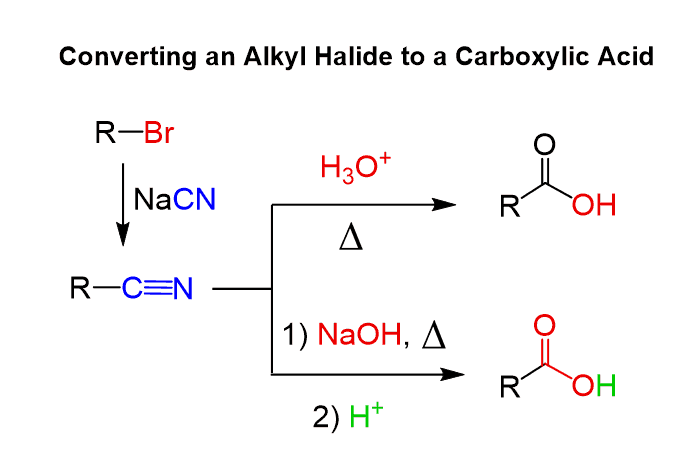
Notice that this approach also allows for adding an extra carbon atom which otherwise requires either an external alkyne for alkylation or an organometallic such as the Grignard reaction.
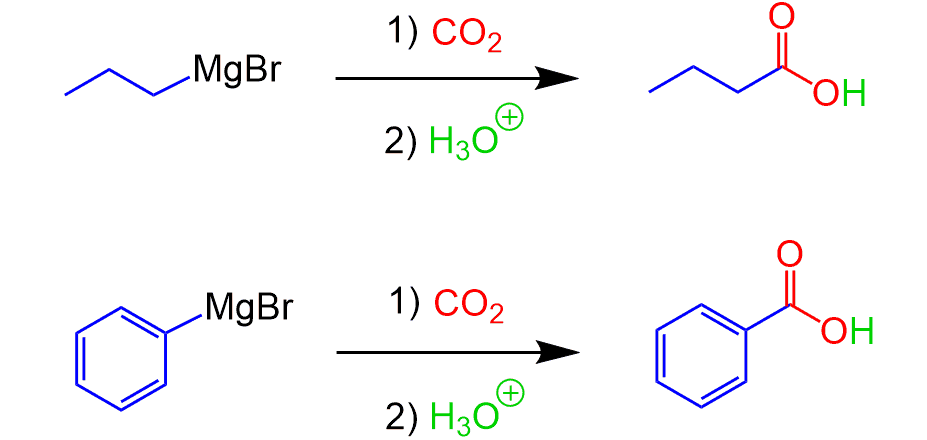
Grignard Reaction with CO2
And talking of Grignard reaction, carbonation of alkyl and aryl magnesium halides can also be used for synthesizing carboxylic acids:
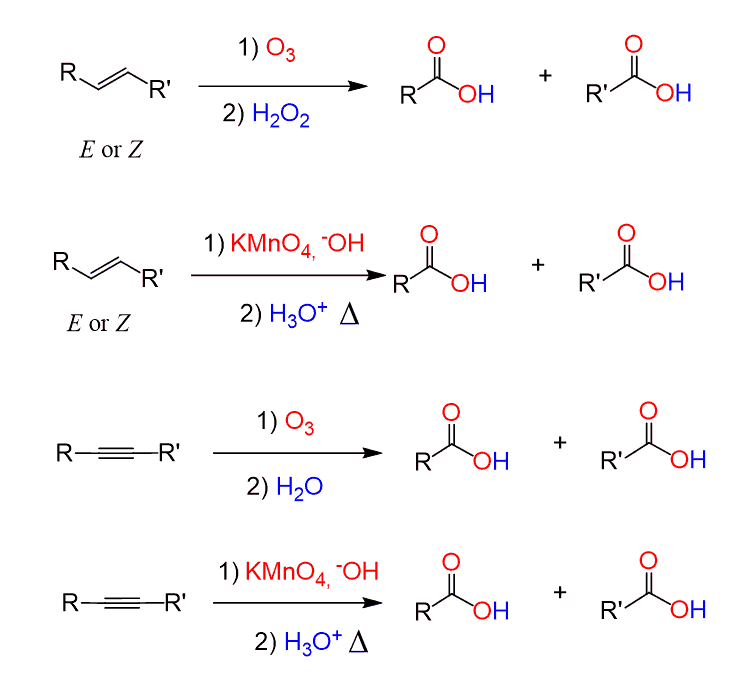
Oxidative Cleavage
Another method for preparing carboxylic acids is the oxidative cleavage of alkenes and alkynes by KMnO4 or ozone:
Check this 45-question, Multiple-Choice Quiz with a 50 min Video Solution on the reactions of carboxylic acids and their derivatives.
Carboxylic Acids and Their Derivatives Quiz
Check Also
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- R2CuLi Organocuprates – Gilman Reagent
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Reduction of Amides to Amines and Aldehydes
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- The Reactions of Nitriles
- Converting Nitriles to Amides
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
- Reactions Map of Carboxylic Acid Derivatives


Is it possible for you to explain the mechanism for Grignard Reaction with Co2 or is there a page that explains it? Thank you.
Yes, exercise 1d under the Grignard Reaction Mechanism has a video solution.