Preparation of Amides
Amides can be prepared from acyl chlorides, esters, and carboxylic acids.
The most straightforward should be the reaction with acid chlorides since they are the most reactive carboxylic acid derivatives, and amines are good nucleophiles too:
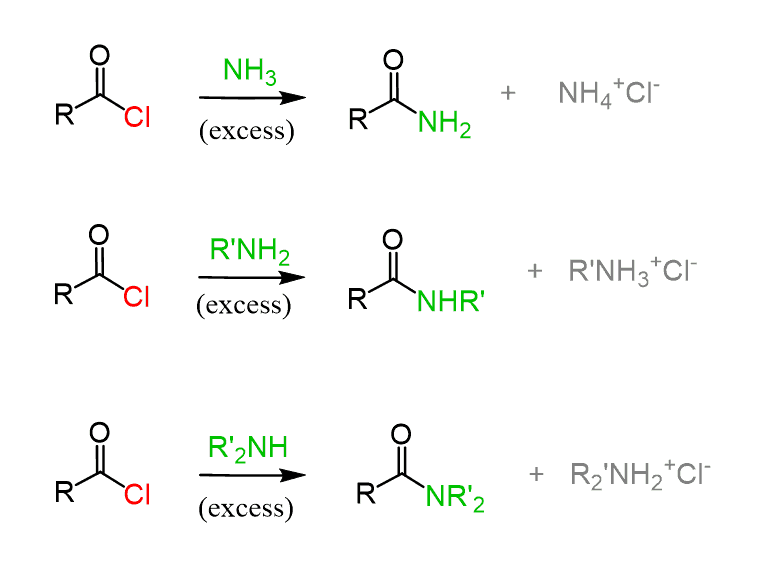
Esters are not as reactive as the acid chlorides and therefore, more forcing conditions are needed to achieve this substitution (aminolysis):

The reaction with carboxylic acids is a little different and there is an important factor to be considered.
Carboxylic acids are strong organic acids (well, yeah…) and amines, besides being good nucleophiles, are also quite strong bases.
So, when these two are mixed, the fast acid-base reaction occurs first forming an ammonium salt, before any nucleophilic substitution reaction:
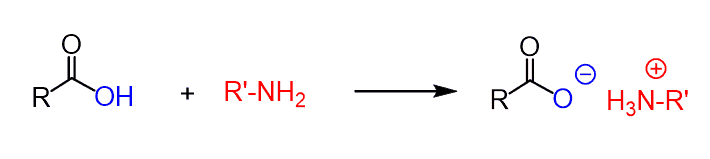
Because of this, it is not practical to prepare an amide by direct reaction of a carboxylic acid and amines. You may still get success by heating the reaction > 100 oC but this also is not the best way around it:
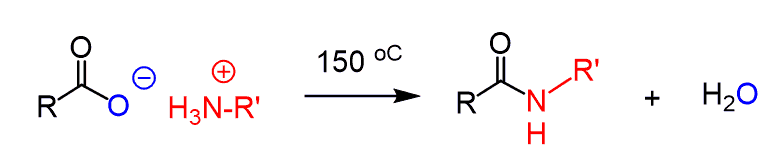
To prepare amides from carboxylic acids under mild conditions, coupling reagents are used. Some of the most common reagents are the dicyclohexylcarbodiimide (DCC), 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBop) and many others.
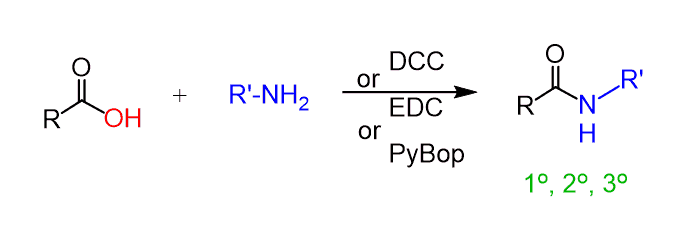
We will discuss the principle of this transformation in a separate article.
Reactions of Amides
We have discussed the structure and the reasons for the low reactivity of amides. They are indeed, the least reactive derivatives of carboxylic acids.
The few but important reactions of amides are listed here as a summary. However, you can find their mechanisms by following the corresponding links.
Hydrolysis of Amides
It is possible to hydrolyze amides with strong acids or bases in harsh reaction conditions:

The mechanism for both hydrolysis reactions involves the two steps of addition elimination reactions.
Esters from Amides
We have seen that esters can be converted to amides since the OR– group of the ester is a better leaving group than the conjugate base of an amine.
This reaction can also be forced in the opposite direction by reacting a large excess of an alcohol with amide:

Just like the hydrolysis, it requires high temperatures and strong acids. Therefore, there is little practical application of this method.
Reduction of Amides
Amides can be reduced to amines with LiAlH4
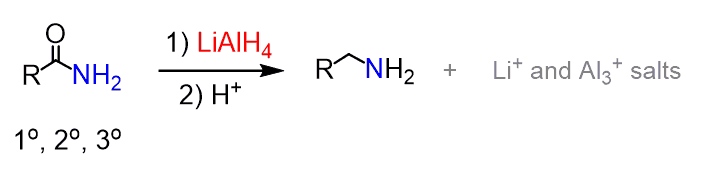
All the above-mentioned reactions pertain to cyclic amides (lactams as well):

Dehydration of Amides to Nitriles
We have seen that nitriles are very useful in organic synthesis as, unlike amides, there is a variety of important reactions they undergo. The good news is that if you have a bottle of a primary amide and need to do some synthesis, you can convert it to a nitrile with a dehydration agent such as SOCl2, P2O5, or POCl3:

Amides to Aldehydes and Ketones
The direct conversion of primary amides to aldehydes is challenging, so we can convert them to nitriles and reduce the nitrile to aldehyde with DIBAL.
The bulkiness of the alkyl groups prevents the second hydride addition, and we can quench the reaction by hydrolyzing the intermediate iminium salt to an aldehyde.

More details about the hydrolysis of imines and enamines are covered here.
Tertiary amides can be reduced directly to aldehydes using the alkyl borane Sia2BH (Disiamylborane (bis(1,2-dimethylpropyl)borane)). Like in the reduction of nitrile to aldehydes, the second hydride addition does not occur, and the hydrolysis of the boronic ester intermediate gives the corresponding aldehyde as the final product.
It is worth mentioning that another alkylborane 9-BBN reduces tertiary amides to primary amines:
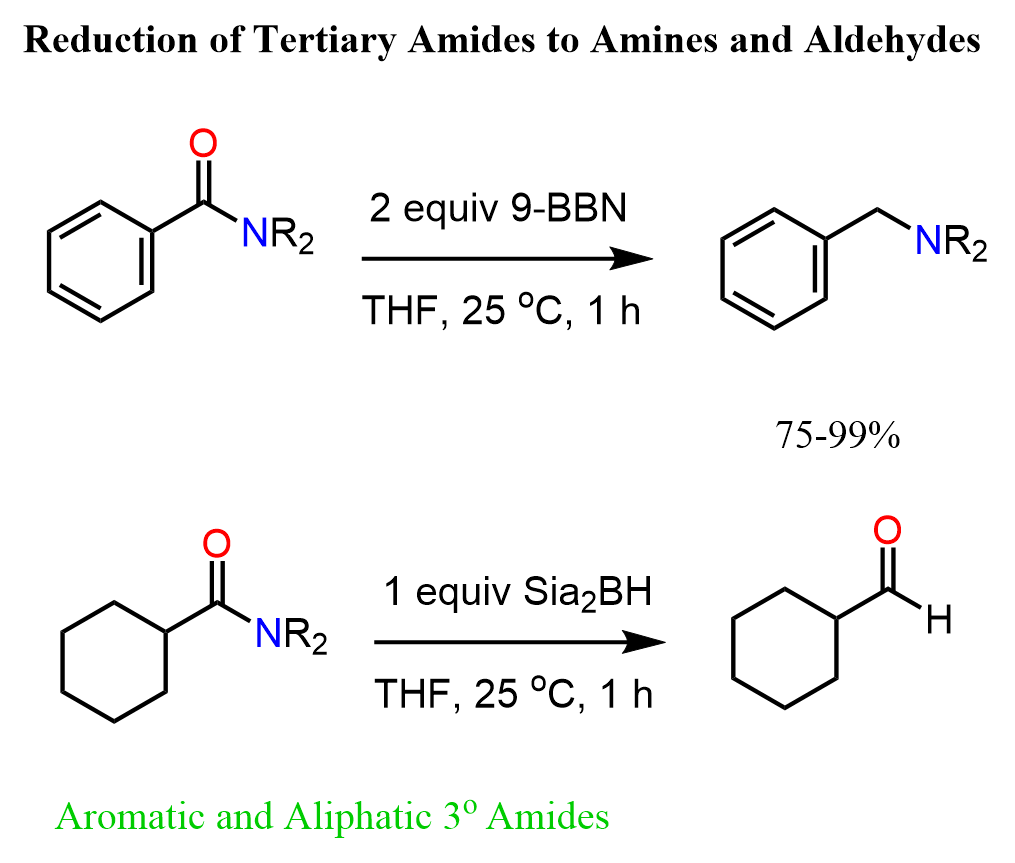
The reduction with alkylboranes is challenging for primary and secondary amides because of the formation of C-O and C-N boronic esters.
Here is the original article for further reading: Tetrahedron Letters, Vol. 38, No. 10, pp. 1717-1720, 1997.
The possibility of converting primary amides to nitriles allows for their indirect conversion to ketones as well:

Check the article about reactions of nitriles for organometallics for more details.
Let’s summarize all the strategies we discussed for converting amides to aldehydes and ketones in one synthetic scheme:
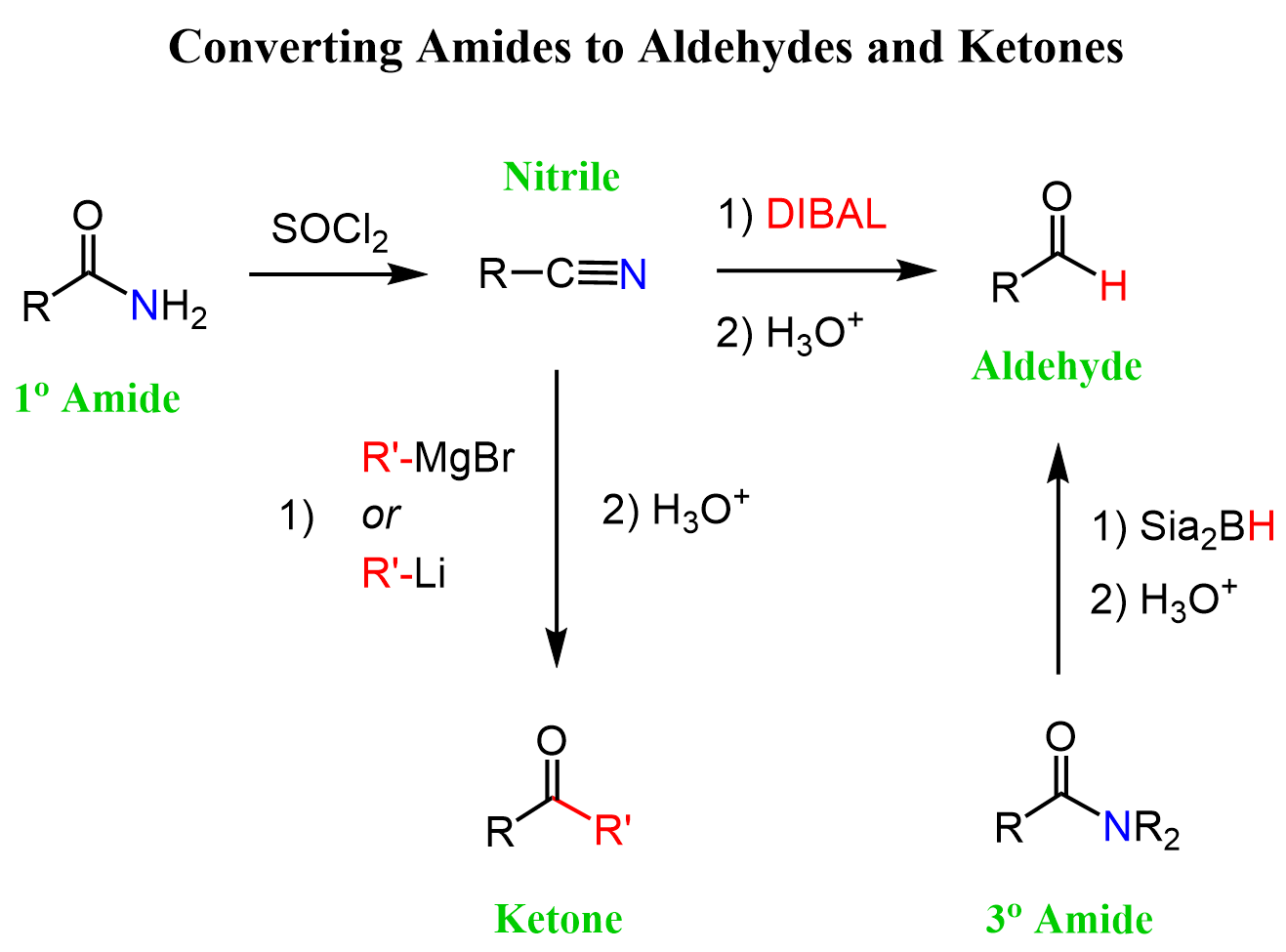
You can find more details about converting amides to aldehydes and ketones here.
Here is the summary for the preparation and most common reactions of amides, which you can use to work on the practice problems:

Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- The Addition-Elimination Mechanism
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- R2CuLi Organocuprates – Gilman Reagent
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Reduction of Carboxylic Acids and Their Derivatives
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Reduction of Amides to Amines and Aldehydes
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- The Reactions of Nitriles
- Converting Nitriles to Amides
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
- Reactions Map of Carboxylic Acid Derivatives
