Amides
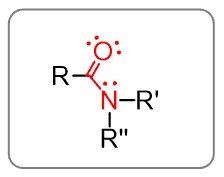
As you know from the functional groups, amides are the “combination” of the carbonyl and amines:
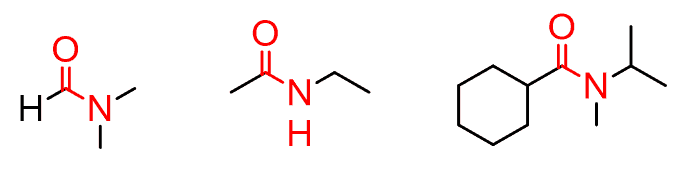
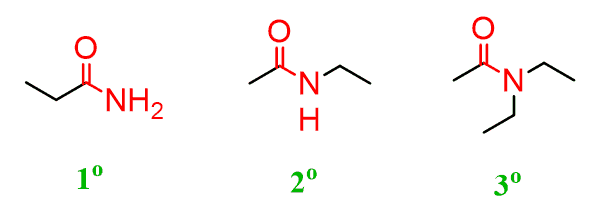
Depending on the number of carbons connected to the nitrogen, we have primary, secondary and tertiary amides. Notice that the carbonyl carbon is also counted:
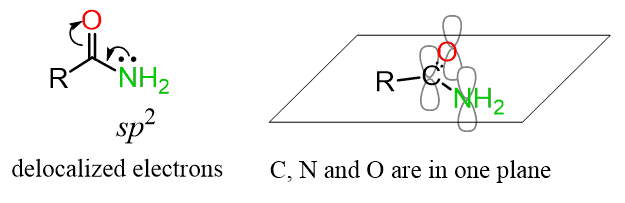
Both the oxygen and the nitrogen as sp2-hybridezed which makes the lone pairs on the nitrogen delocalized, in resonance with the p orbital of the C=O double bond:
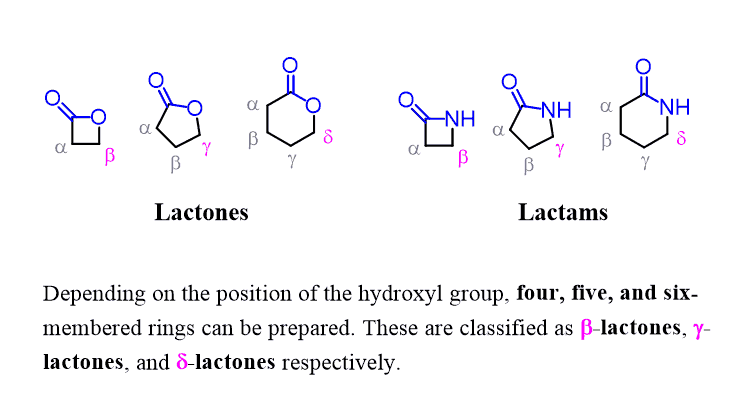
There are also cyclic amides called lactams. Just the lactones, these are classified by ring size as γ-lactams (five-membered lactam ring), β-lactams (four-membered lactam ring), etc:
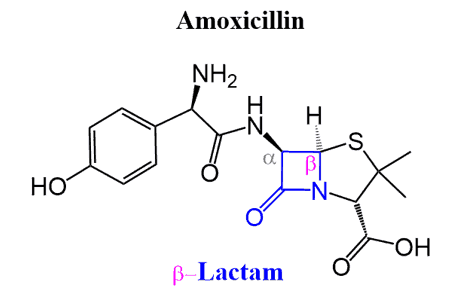
β-lactams are the building unit of a large family of penicillin-type antibiotic:
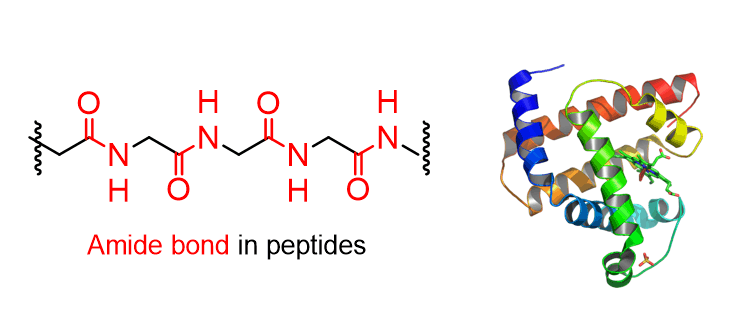
Stability of the Amide Bond
The amide bond (amide linkage, peptide bond) is the linking unit in proteins which are polymers of amino acids. And, by definition, this bond must be stable in aqueous cellular environment and in other biological systems:
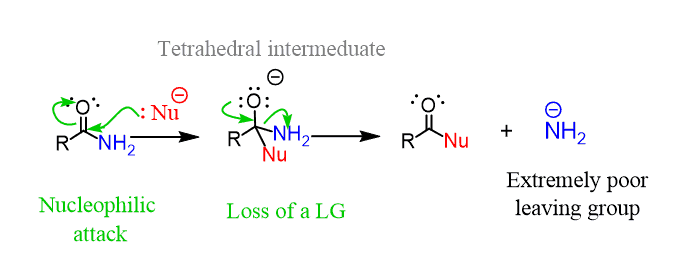
There are two main reasons for this. First is the fact that they have the poorest leaving group (–NH2, RNH–, R2N–) because the pKa of amines ranges from 32-38 and their conjugate bases are very strong bases and, therefore, poor leaving groups:
Second, carbon and nitrogen are in the same row of periodic table which allows for a perfect overlap of their p orbitals which significantly reduces the partial positive charge on the C=O carbon. As a result, it is less electrophilic and less susceptible to a nucleophilic attack:

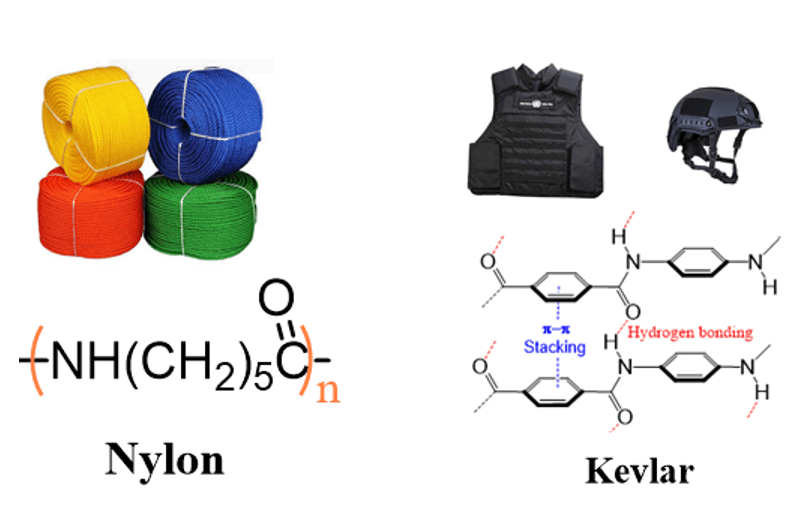
The applications of amides are not limited to biological systems only. In addition to having the exceptional properties as a covalent bond, the peptide bond displays strong intermolecular interactions such as the hydrogen bonding of the carbonyl groups. This, together with the aromatic π-π stacking interactions, are indispensable for making Kevlar (used for production of bullet proof materials), Nylon, and other synthetic polymers:
So, it is not a surprise that amides are the least reactive carboxylic acid derivatives in nucleophilic acyl substitution reactions. Nevertheless, they do participate in some important reactions which we need to discuss.
Check Also
- Carboxylic Acids and Their Derivatives Practice Problems
- Preparation of Carboxylic Acids
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Amides Preparation and Reactions Summary
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles

thanks sir
i am from india
Welcome, Shivansh.