Nitriles can be reduced to primary amines when treated with LiAlH4 or to aldehydes when a milder reducing agent such as DIBAL is used.
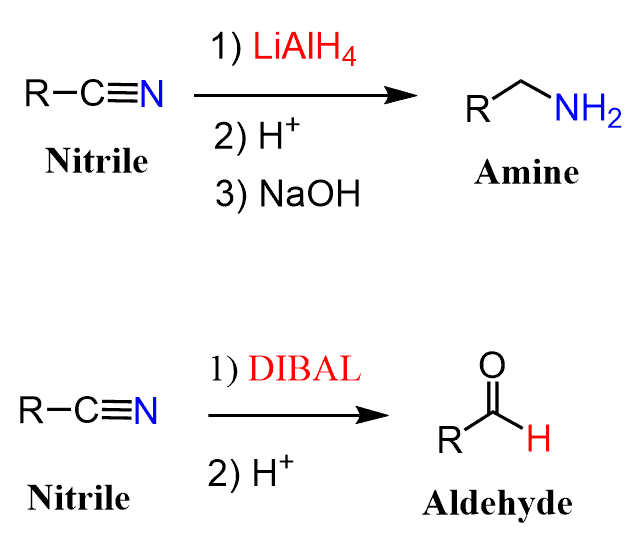
Just like any other reduction reaction, an acidic or aqueous workup is needed to get rid of the ionic intermediates. If the final product of the reaction is an amine, then it is usually treated with a hydroxide to deprotonate and isolate it in a neutral amine form.
The Mechanism of Nitrile Reduction with LiAlH4
The reaction starts with q nucleophilic addition of a hydride ion. This forms an imine salt, which undergoes another nucleophilic addition by AlH3, producing a highly reactive derivative of an amine:

Both N-Al and N-Li bonds in this derivative are very polar and quickly react with water, forming the new N-H bonds of the primary amine:

Notice that the arrow starting from the N-Li bond indicates the electrons forming an N-H bond and not Li-H. The lithium is almost ionic (positively charged) and reacts with the -OH forming LiOH. Likewise, in the second step of the workup, the arrow starting from the N-Al bond forms a new N-H bond and Al(OH)H2.
In summary, this is what we have as a plausible mechanism for the reduction of nitriles to primary amines:

The Mechanism of Nitrile Reduction by DIBAL
DIBAL is a milder reducing agent than LiAlH4, and it can be used for the selective reduction of esters and nitriles to aldehydes.
The reaction again starts with a hydride addition to the C-N triple bond, forming an iminium anion. The difference between DIBAL and LiAlH4 is that DIBAL is not powerful enough to perform another hydride addition to the negatively charged intermediate.
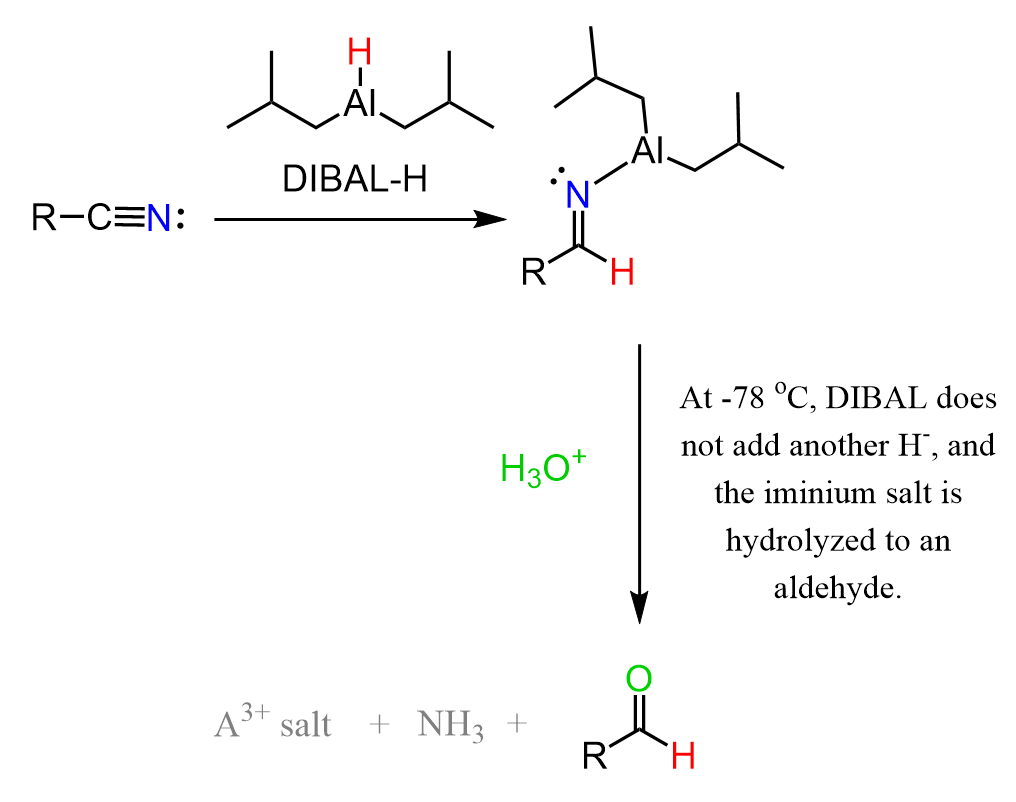
And this is the advantage of DIBAL because, after the first hydride addition, we can quench the reaction by aqueous workup, which hydrolyzes the imine to an aldehyde.
Combining these two parts, here is what we have for the mechanism of Nitrile reduction to an aldehyde by DIBAL:
More details about the hydrolysis of imines and enamines are covered here.
You may not need this in practice as an undergrad, but let me mention that these reactions are nowhere near as smooth and ideal as they appear on paper. Very often, you may end up reducing these derivatives to alcohols and oxidizing them to aldehydes since DIBAL will do a complete reduction as LiAlH4 does.
Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- R2CuLi Organocuprates – Gilman Reagent
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Reduction of Amides to Amines and Aldehydes
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- The Reactions of Nitriles
- Converting Nitriles to Amides
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
- Reactions Map of Carboxylic Acid Derivatives

Plz give me a detail mechanism of reduction of methylcyanide in the presence of Ni
Amine is written as alcohol in first reaction with LiAlH4.
Thank you! Fixed.
Professor: Will DIBAH also reduce carboxylic acids to aldehydes or to alcohols?
It can reduce carboxylic acids, but: 1) keep in mind that at least three equivalents would be needed to obtain the alcohol because reducing agents, including DIBAL, are sources of hydride ion which is a base, so for carboxylic acids, the first equivalent goes for the deprotonation of the acid. 2) Stopping the reduction at an aldehyde or ketone is not as easy as it is given in undergraduate textbooks, so very often, the product is going to be the corresponding alcohol. LiAlH4 is the standard choice for reducing carboxylic acids to alcohols.
professor: when DIBAL-H reduce cyanide to aldehyde, then what are the by products obtained from DIBAL-H. some sources showing Ammonia and aluminum salts (but what are the salt (name)s it will results) plz clarify?