Both Grignard and organolithium reagents can be used to convert nitriles to ketones.

The new C-C bonds are formed by nucleophilic addition of the organometallic reagents to the polar C-N triple bond. After protonation of the resulting anion, an imine is formed which is then hydrolyzed to a ketone.
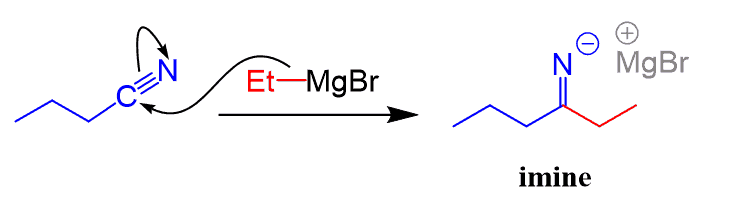
Notice that this is different from the Grignard reaction of aldehydes, ketones, esters and acid chlorides where the major product was an alcohol.
The reason for this difference is that the negatively charged imine cannot go another nucleophilic addition since that would place two negative charges on the nitrogen.
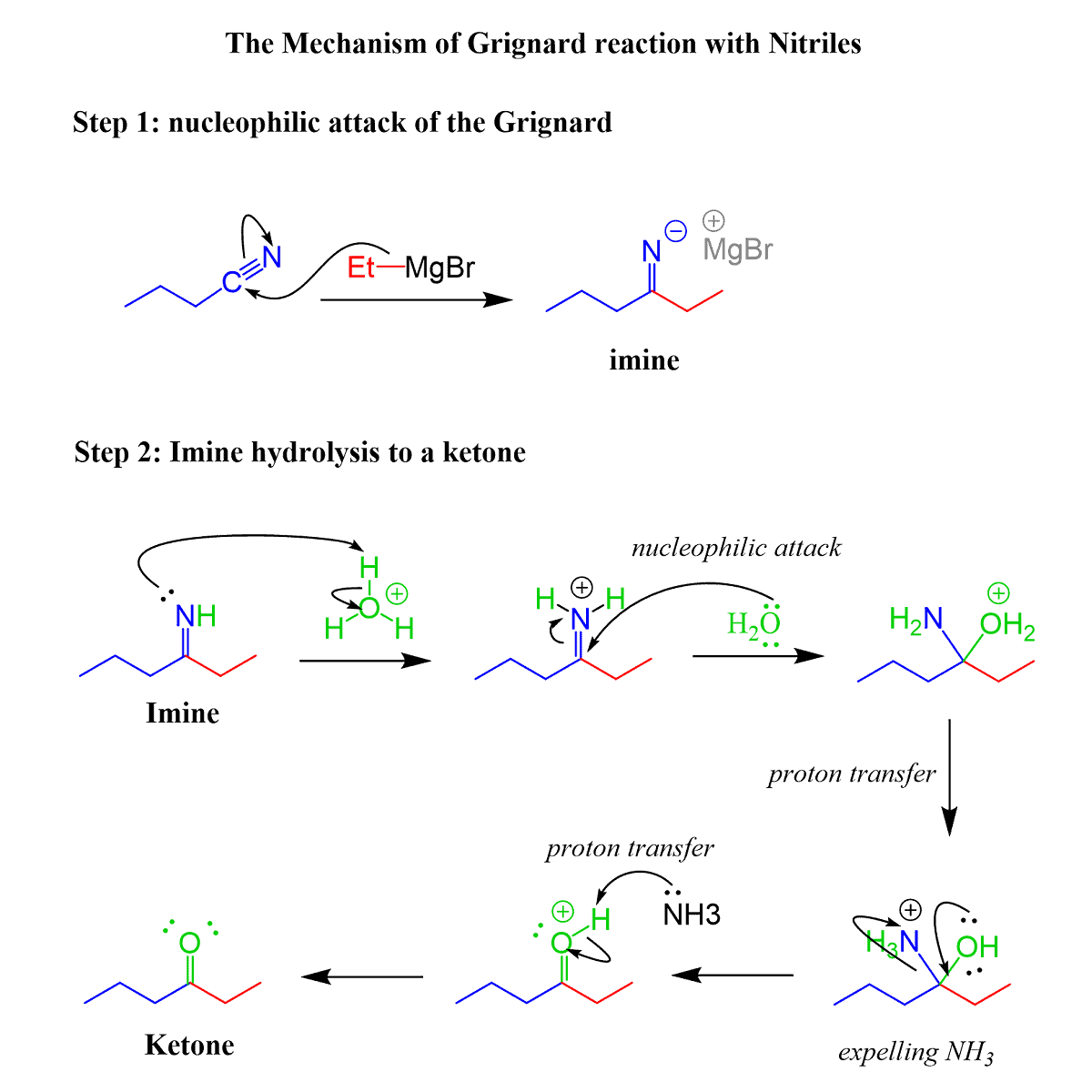
The Mechanism of Nitrile Reaction with Grignard Reagents
After the nucleophilic addition of the alkyl group to the C-N triple bond, water or acidic workup is performed which quenches the organometallics and hydrolyzes the imine into the corresponding ketone:
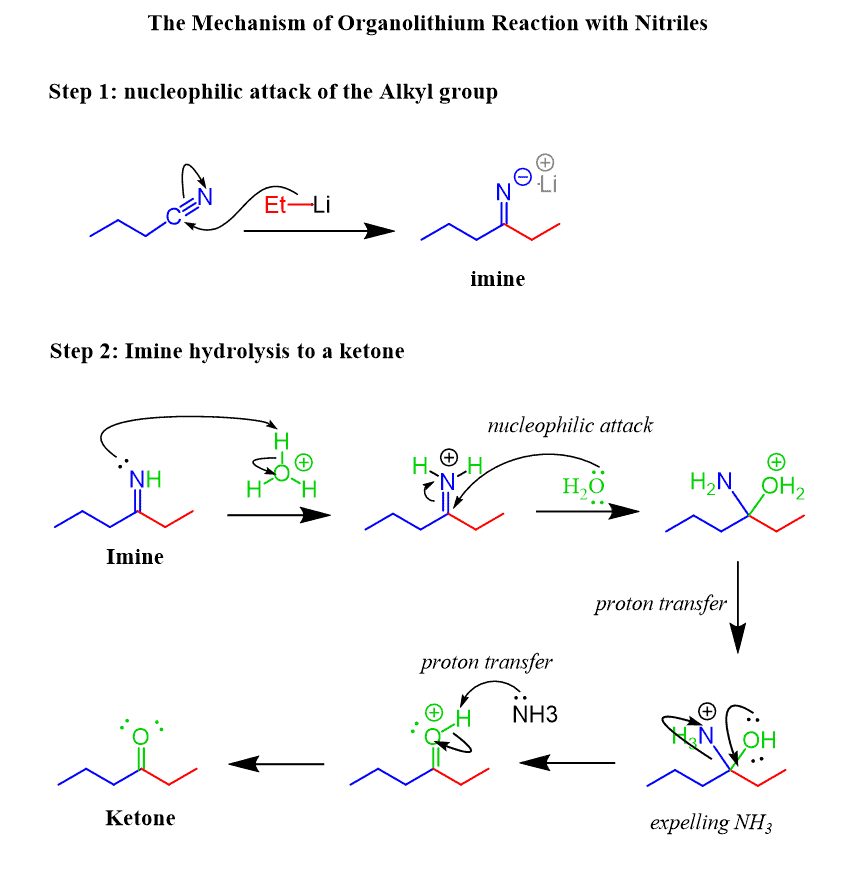
The Mechanism of Nitrile Reaction with Organolithiums
In an identical to the Grignard reaction mechanism, nitriles are converted to ketones with organolithiums:
Check Also
Practice
1.
Determine the product in each of the following reactions:
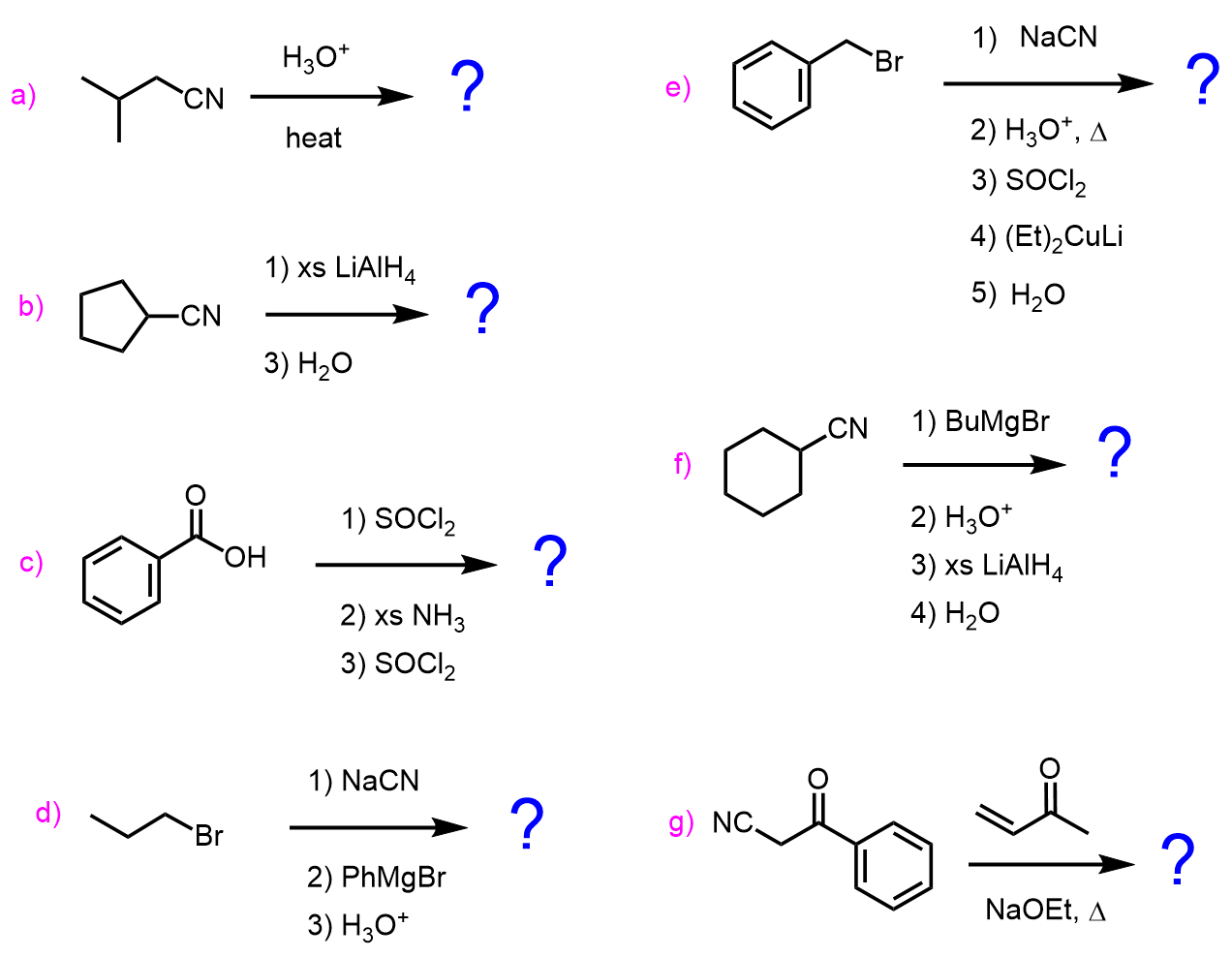
a)
Answer
This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
b)
Answer
This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
c)
Answer
This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
d)
Answer
This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
e)
Answer
This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
f)
Answer
This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
g)
Answer
This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
2.
Identify the reagents you would use to achieve each synthetic transformation:
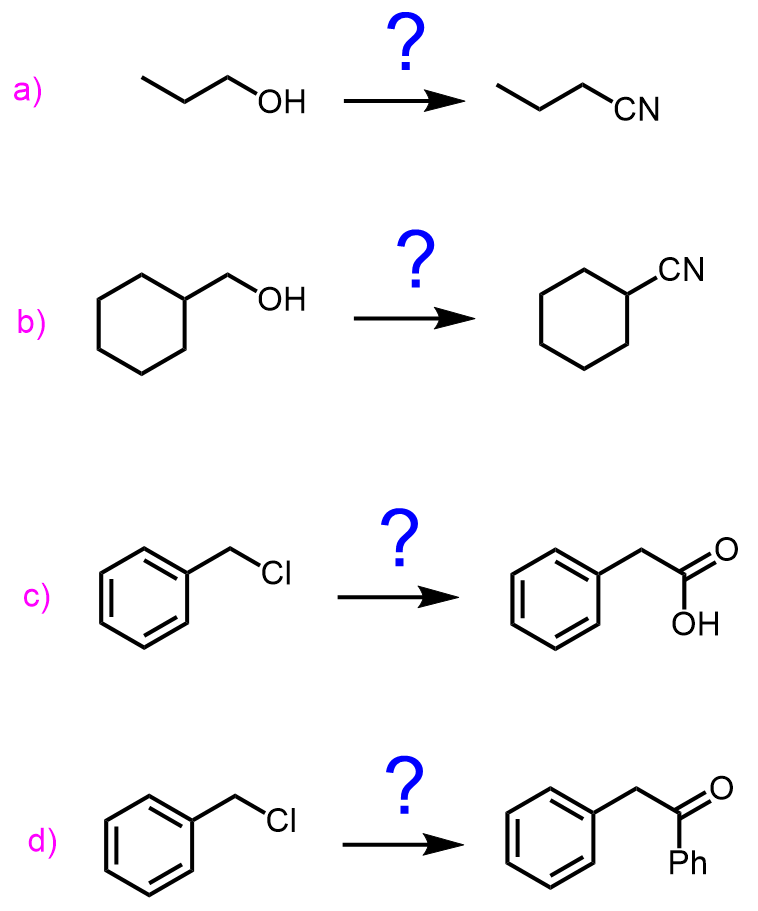
a)
Answer
This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
b)
Answer
This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
c)
Answer
This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
d)
Answer
This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
3.
Propose a mechanism for the following synthetic transformation:
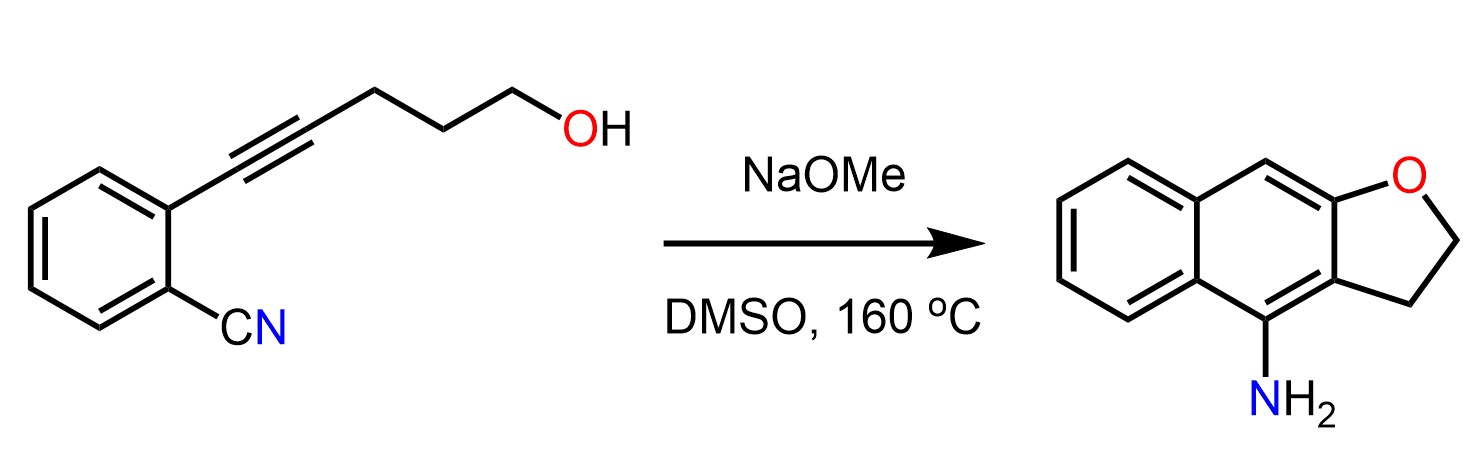
Answer
This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
4.
Compare the two reactions below and explain the role of solvents in having such different products.
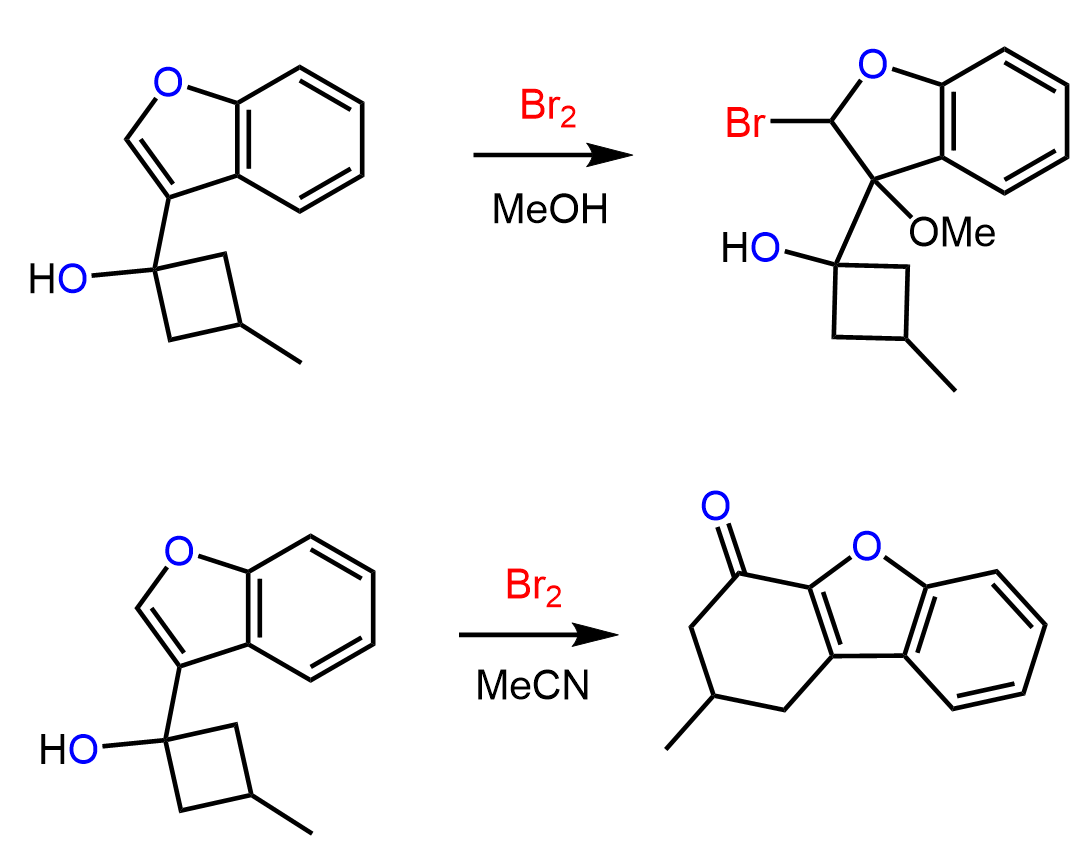
Answer
This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.










indeed you have given to us a more closely and most frequent used mechanism. thank you.
is there any other condition that is required for this reaction? for example the reaction between grignard reagent and aldehyde or ketone requires the presence of dry ether.
That is correct, the Grignard, and any other reaction involving organometallics, requires dry condition, and dry ethers are most often used as solvent. Keep in mind that even though the ether forms complexes with the organometallics, it is not part of the reaction but rather just a solvent.
I had to do this reaction not too long ago. It is a bit more complex than this but there is not a great deal of recent literature.
1) It is much slower than attack on carbonyl.
2) Grignard base can deprotonate the beta position – needs to be added slowly, in the order Grignard->nitrile (supposedly), and cold or side products dominate.
3) It isn’t an ion. It is a complex: N…Mg. Relevance being that it can’t be counted on to form if the complex isn’t stabilised
2 fold excess of Grignard to form a Mg-dimer complex apparently works well. Though X=Cl apparently works well enough with 1 eqv. Big differences between eg. THF and Et2O also.
It is a pain compared to Grignard rxn, basically. But it works, in some yield.
PS. you have an extra double bond in the second to last structure
Thanks for sharing – all great points.
Indeed, the complexity of these reactions and their experimental challenges are quite different from what we draw on paper. It is just hard to write an article that suits the needs of undergrad and higher levels. I can’t imagine how much time it will take me to address them in a single post and, at the same time, not destroy the motivation of an undergraduate student trying to learn the essentials of the reaction🙂 We do address, the competition of basicity and nucleophilicity starting from the E2/SN2 business, but it is still disheartening to take off points for a nicely designed synthesis because the acid-base reaction was ignored in favor of a nucleophilic attack.
Addressing these details in the comments is a great idea and may help those who need to go a little further, so please add and share more.
PS – image fixed.