We have seen that the product of the aldol reaction is a β-hydroxy carbonyl compound:

One can look at it as an alcohol, and what is interesting about this alcohol is that it undergoes an elimination when heated in presence of a base or an acid:

This two-step process is the aldol condensation and the final product of it is an ɑ, β-unsaturated carbonyl compound.
The E1CB Mechanism in Aldol Condensation
Let’s sum this one more time – the aldol addition reaction is the reaction between the enolate and the carbonyl producing an β-hydroxy carbonyl compound. This compound is then converted into an ɑ, β-unsaturated carbonyl compound via an elimination reaction. The two steps together are referred to as the aldol condensation reaction.
Now, what is the mechanism of this elimination? We don’t normally expect an alcohol to undergo an elimination reaction since the OH is a poor leaving group. It only happens through and E1 by using a strong acid or by converting the OH into a good leaving group such as a mesylate, tosylate or a simple halide.
So, the fact that elimination works with a moderate base such as NaOH, indicates two things; 1) that the OH is, for some reason, kicked out and 2) the hydrogen between the OH and C=O group is removed as judged from the position of the double bond in the product.
Let’s address these by starting from the hydrogen. The only difference in β-hydroxy carbonyl compounds compared to regular alcohols is the presence of the carbonyl group and makes the hydrogen more acidic:
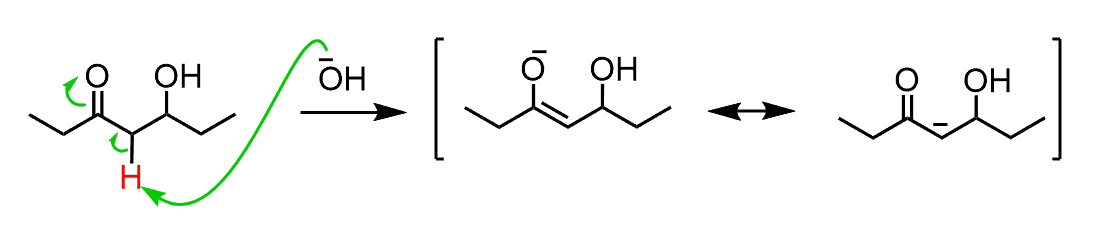
However, carbonyl compounds are not deprotonated by hydroxides to a significant degree either while these eliminations have quite high yields. And this has to do with the OH group, which after being expelled by the π electrons forms am energetically favored conjugated system:

Once the ɑ, β-unsaturated carbonyl compound is formed, it shifts the equilibrium of the whole process to the right as it brings in an irreversible step.
Unlike the aldol addition reaction, the aldol condensation does not generally occur at room temperature. Just like other elimination reactions, it is entropically favored at higher temperature (~100 oC)
Some exceptions are when the final product is a conjugates system of multiple π bonds since this gives additional stability and serves as a driving force for the reaction.
For example, acetophenone undergoes aldol condensation so readily that the intermediate β-hydroxy carbonyl compound is not isolated as it immediately transforms into a highly conjugated system:

The aldol condensation is also efficient for ketones which at lower temperature establish an unfavorable equilibrium of an aldol addition reaction:
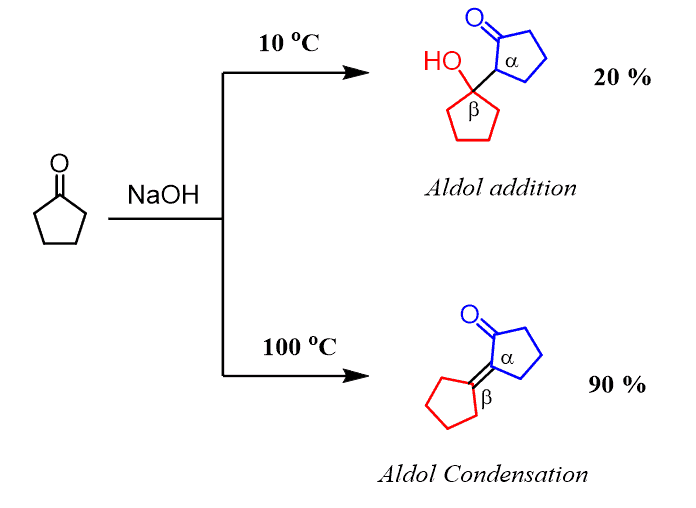
This, again, is explained by the formation of a conjugated system of π electrons which are not present in the aldol addition product.
The Shortcut of Aldol Condensation Reactions
Remember, we talked about the strategy for quickly determining the product of aldol addition reactions. In short, you needed to draw the “V” shape of the β-hydroxy carbonyl unit and add the remaining alkyl groups based on the aldehyde or the ketone being reacted:

Now, to determine the product of an aldol condensation, you can follow the steps shown above, and in the end, remove the OH by adding a C=C double bond conjugated to the carbonyl.
Alternatively, align the two reacting carbonyls such that the ɑ carbon of the nucleophile is pointing to the C=O group of the electrophile and connected them with a double bond by removing the C=O oxygen:

If this made sense so far, try solving a good set of aldol condensation problems here:
https://www.chemistrysteps.com/aldol-reaction-practice-problems/
Check Also
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Dieckmann condensation – An Intramolecular Claisen Reaction
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- Enolates in Organic Synthesis – a Comprehensive Practice Problem
