In the previous post, we talked about the acetoacetic ester synthesis which is used for preparing ketones having one or two alkyl groups on the ɑ position:
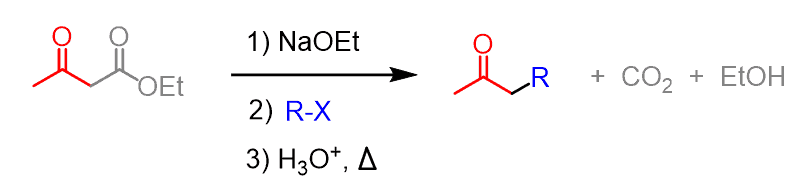
Another way to look at this reaction is to say that it is used to prepare ketones form alkyl halides. And a similar transformation can be achieved by using the malonic ester synthesis. The only difference is that the final product is a carboxylic acid instead of the ketone obtained in the acetoacetic ester synthesis:

You may wonder how it is similar if the final product is different. It is similar conceptually and mechanism-wise. Just like before, we are using a dicarbonyl compound to lower the pKa of the ɑ hydrogen, and at the end, heating up the reaction to get rid of the ester groups via decarboxylation.
The only difference is that the additional ester group here is converted into a carboxylic acid during the acidic treatment for the decarboxylation.
As an example, let’s illustrate how malonic ester and 1-iodopropane are used to prepare pentanoic acid:

In the first step, the sodium ethoxide deprotonates the ester forming a doubly stabilized carbanion which then serves as a nucleophile attacking the alkyl halide. The acidic work-up hydrolyzes the ester groups into carboxylic acid which undergo a decarboxylation upon further heating.
Notice the use of sodium ethoxide as a weaker base than LDA which is used in the direct alkylation of ketones. And this is what the two carbonyl groups to together since dicarbonyl compounds are about twice more acidic compared to their analogs with only one electron-withdrawing group:

There are, however, some limitations to the bases you can use, and these are covered in the acetoacetic ester synthesis. In short, it needs to be the same alkyl group as in the malonic ester.
If another alkyl halide is added to the reaction before the hydrolysis and decarboxylation step, double alkylation of the ɑ position occurs:

Now, for the decarboxylation, it occurs by the same mechanism as we discussed in the previous post.
In the first part, the ester groups are hydrolyzed to carboxylic acids which then lose carbon dioxide through a nicely arranged six-membered transaction state:

As always, practice problems on the malonic ester synthesis and alpha carbon chemistry can be found here:
Enolates in Organic Synthesis – a Comprehensive Practice Problem
Check Also
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Dieckmann condensation – An Intramolecular Claisen Reaction
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- Enolates in Organic Synthesis – a Comprehensive Practice Problem

