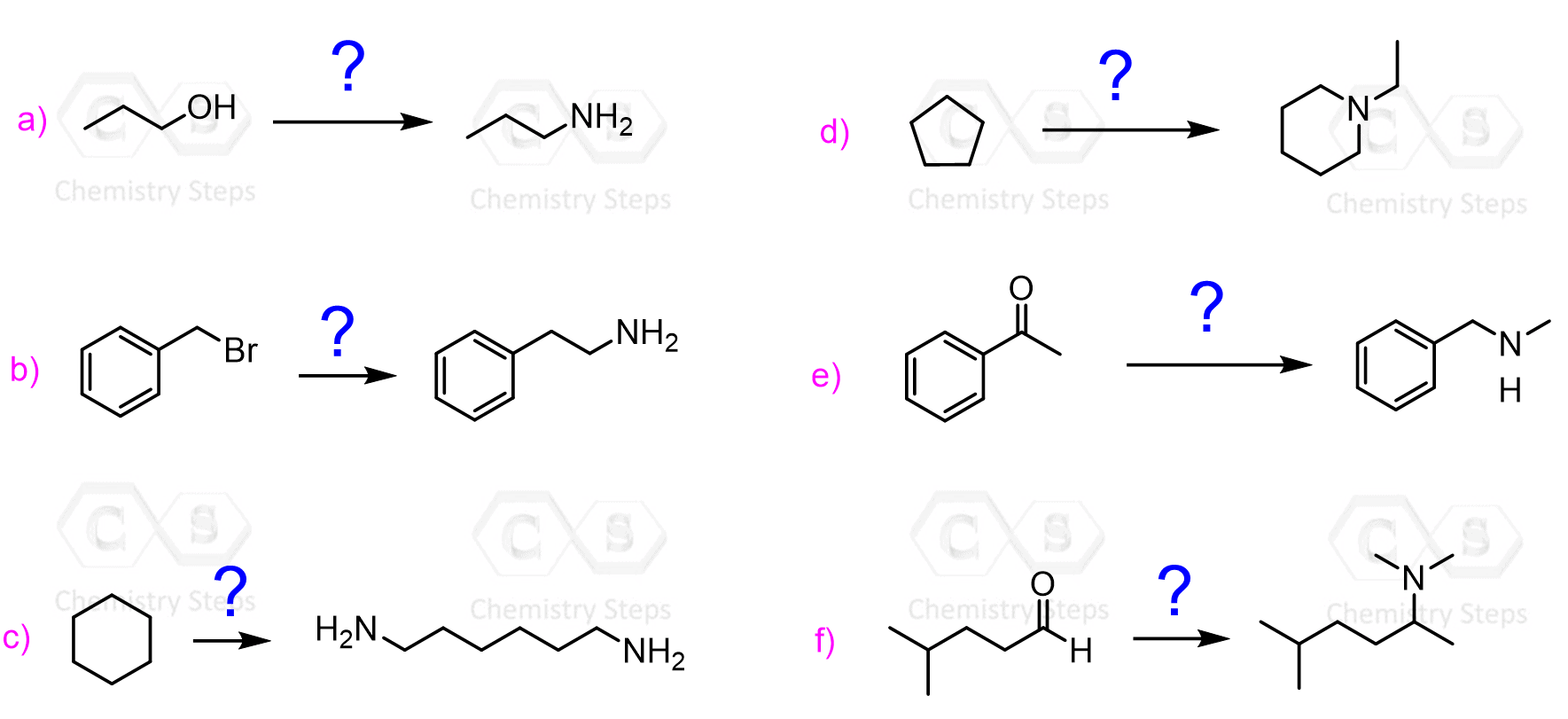
A variety of reactions that we have seen earlier can be used for preparing amines. The most straightforward would look like an SN2 reaction of an alkyl halide with ammonia or an amine:

The main drawback of this method is the polyalkylation of the nitrogen nucleophile. The problem is that the amine formed by nucleophilic substitution still has a lone pair of electrons; thus, it can serve as a nucleophile or a base reacting with the alkyl halide. This gives a mixture of 1°, 2°, and 3° amines, which limits its application.

In addition to the substitution, the elimination reactions become an issue, too, as the alkyl groups keep adding, resulting in secondary and especially tertiary amines.
Only quaternary ammonium salts cannot react as a base or a nucleophile because they lack a lone pair and the four alkyl groups on the positively charged nitrogen.
You may ask, can’t we use a large excess of the initial amine to prevent the reaction of the product amine? Yes, it is possible; however, it is mostly useful for preparing 1° amines since here we need the relatively inexpensive ammonia.
The Gabriel Synthesis of Primary Amines
The limitation of polyalkylation is overcome by using phthalimide as a bulky amine-containing reagent.

The phthalimide is first deprotonated, forming a good nucleophile to react with the alkyl halide, and in the last steps, the side product is either hydrolyzed like an amide or alternatively, hydrazine is used to liberate the free amine. This is called the Gabriel reaction and you can find more details about it here.
Amines by Reduction of Other Functional Groups
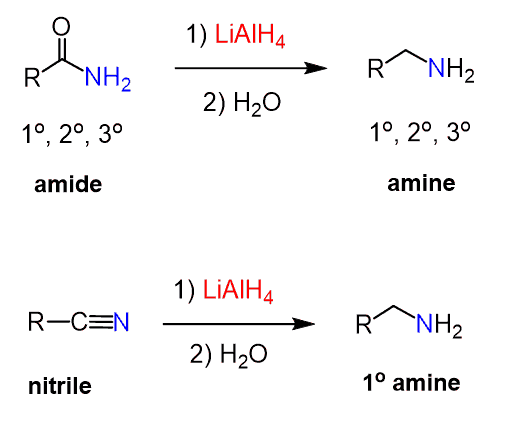
Several groups can be reduced to amines using either hydride ion reductions or catalytic hydrogenation. For example, amides and nitriles can be reduced using lithium aluminum hydride. Depending on the structures, primary, secondary, and tertiary amines can be prepared from amides. Nitriles, on the other hand, will only lead to primary amines:
The advantage of nitriles is the fact that they are derived from the cyanide ion, which is a good nucleophile and can be used to convert an alkyl halide to an amine in two steps:
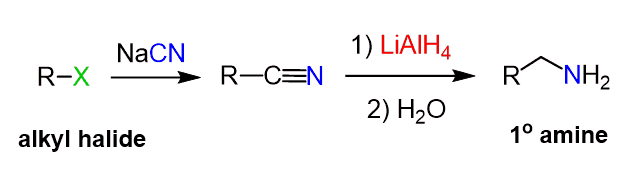
In a similar manner, azide ion can be used as a good nucleophile by reacting with an alkyl halide and later reduced to an amine:
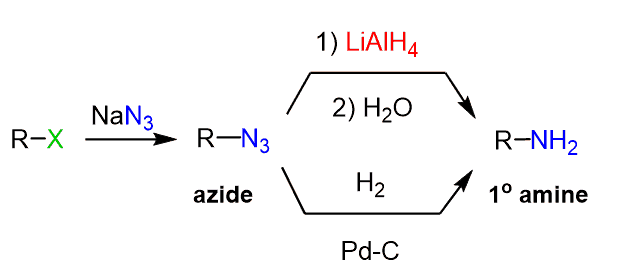
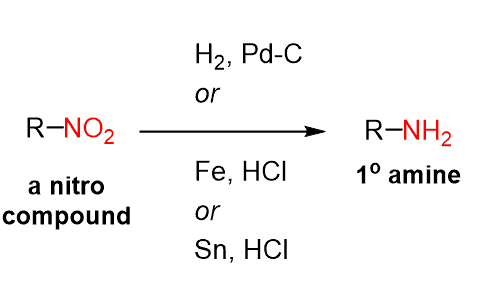
Nitro compounds are efficiently reduced using hydrogenation:
One more important reaction is the reductive amination. Here, an aldehyde or a ketone is converted into an imine by reacting with a primary amine, which is then reduced to an amine with sodium cyanoborohydride (NaCNBH3).
Secondary amines react with aldehydes and ketones, forming a reactive intermediate, iminium ion, which, being more reactive than carbonyls, is quickly reduced to a tertiary amine.

Hofmann and Curtius rearrangements
We have seen above that using a cyanide nucleophile allows for the preparation of an amine with an extra carbon atom in the chain.
Can we shorten the carbon chain when preparing an amine?
One useful strategy for preparing primary amines involves rearrangement reactions that remove a carbon atom from a starting material. Two classic examples are the Hofmann rearrangement and the Curtius rearrangement. In the Hofmann rearrangement, a primary amide reacts with bromine and a strong base (usually NaOH) to yield a primary amine with one fewer carbon. The key step involves a 1,2-shift through an isocyanate intermediate, which is hydrolyzed to the final amine product. Similarly, the Curtius rearrangement starts with an acyl azide, which upon heating releases nitrogen gas and rearranges to form an isocyanate. In the presence of water, this also gives a primary amine.
This little chart should help you visualize and compare the Hofmann and Curtius rearrangements:
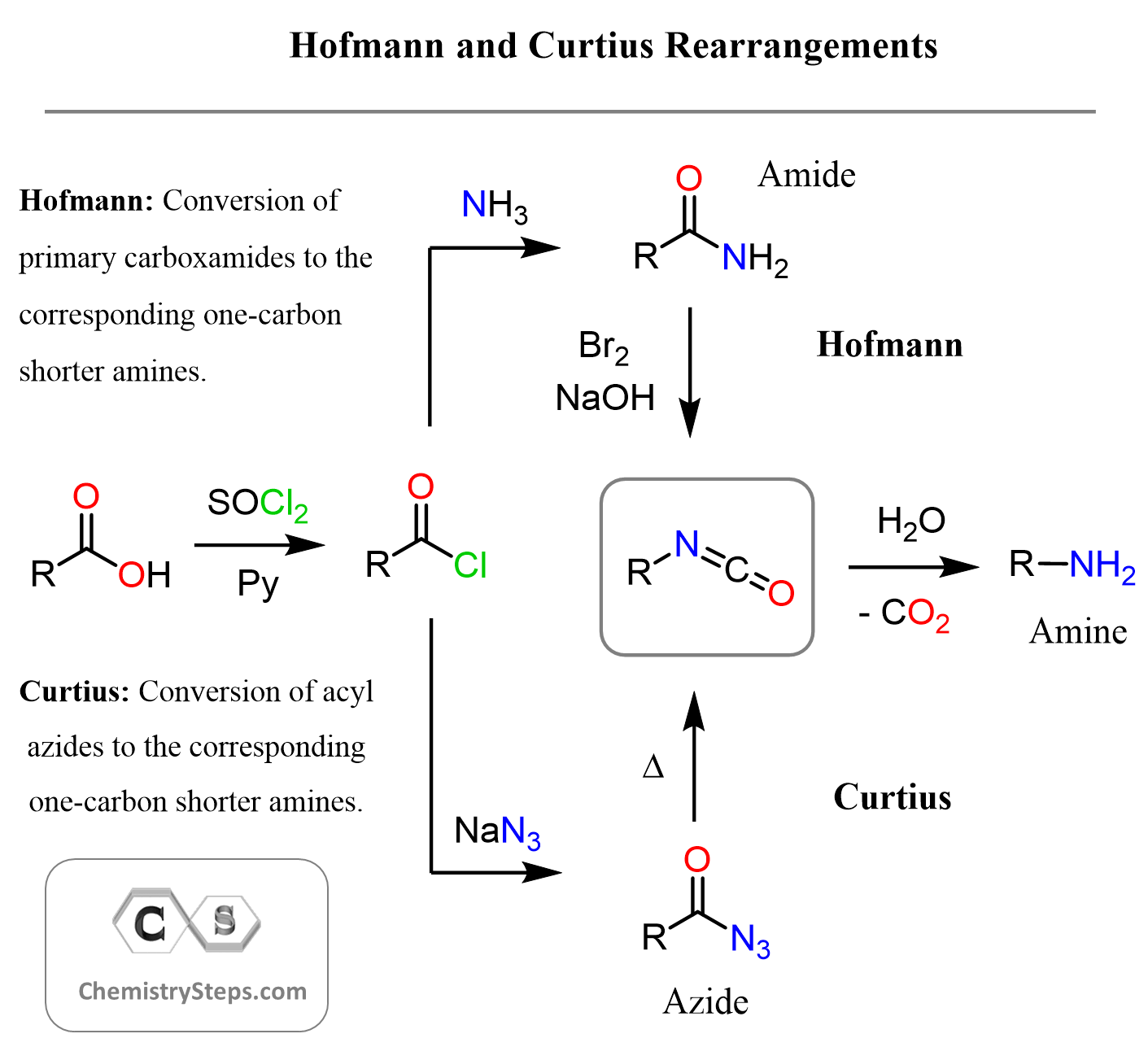
Both reactions are valuable for shortening carbon chains and introducing nitrogen into molecules, and they differ mainly in their starting materials and reaction conditions. Check the linked articles for more information and the mechanism of each reaction.
The Ritter Reaction for Preparing Amines
Although the Ritter reaction is not covered in many textbooks, it is a great combination of principles and reactions, such as carbocations, loss of a leaving group, nucleophilic addition, and hydrolysis, so you should be good at using it for a variety of functional group transformations without violating what you are allowed to use in your assignments.
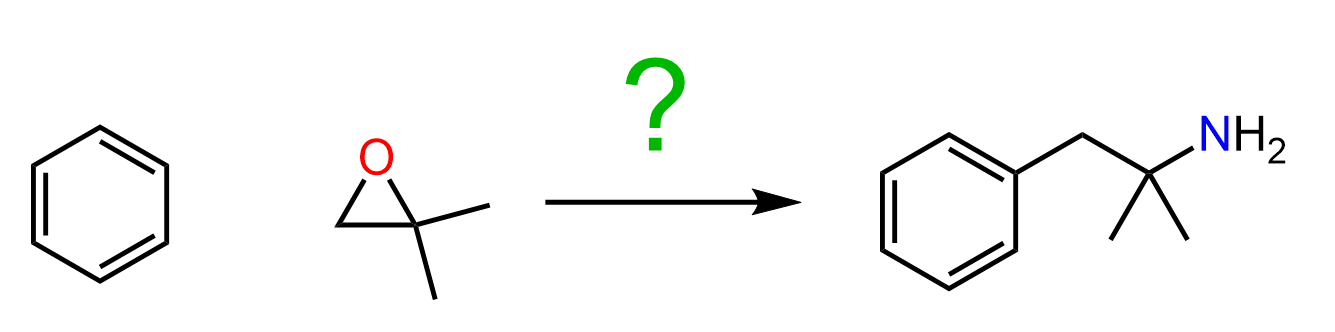
The Ritter reaction can be used for preparing amines from alkenes, alkyl halides, and alcohols. All of these are used as the source of carbocation that is attacked by a nitrile, followed by hydrolysis to obtain an amide, which can further be hydrolyzed into the needed amine.
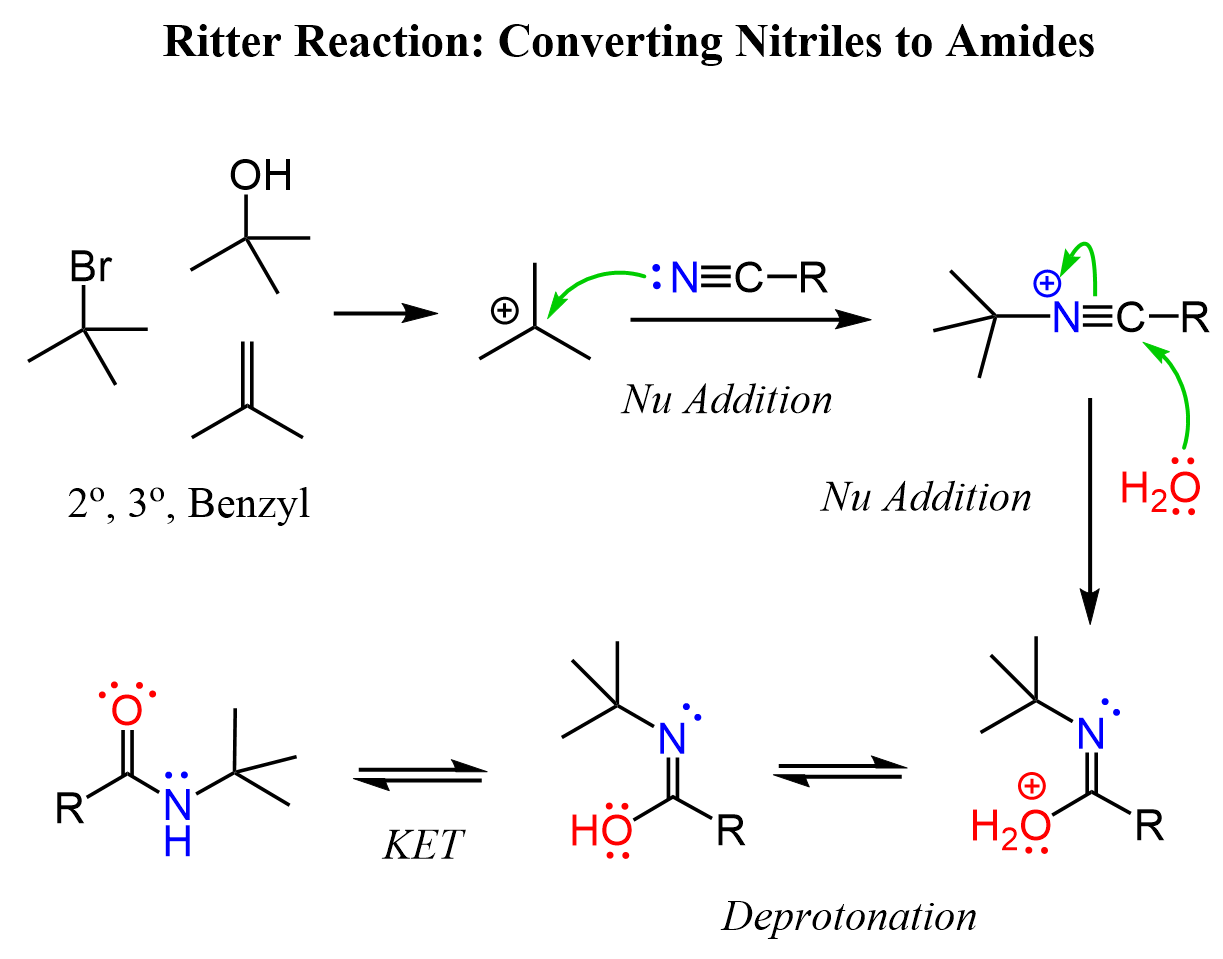
After the nucleophilic attack, the carbon-nitrogen triple bond is activated, and a nucleophilic attack of water occurs, as we have seen in the hydrolysis of nitriles. The nucleophilic addition of water forms an imidic acid intermediate, which tautomerizes to the corresponding amide. What we talked about so far is a nice way of converting, for example, nitriles to amides.
Now, amides can also be hydrolyzed under acidic and basic conditions to the corresponding acid and the amine, so we can construct a summary scheme for converting alcohols to amines using the Ritter reaction.
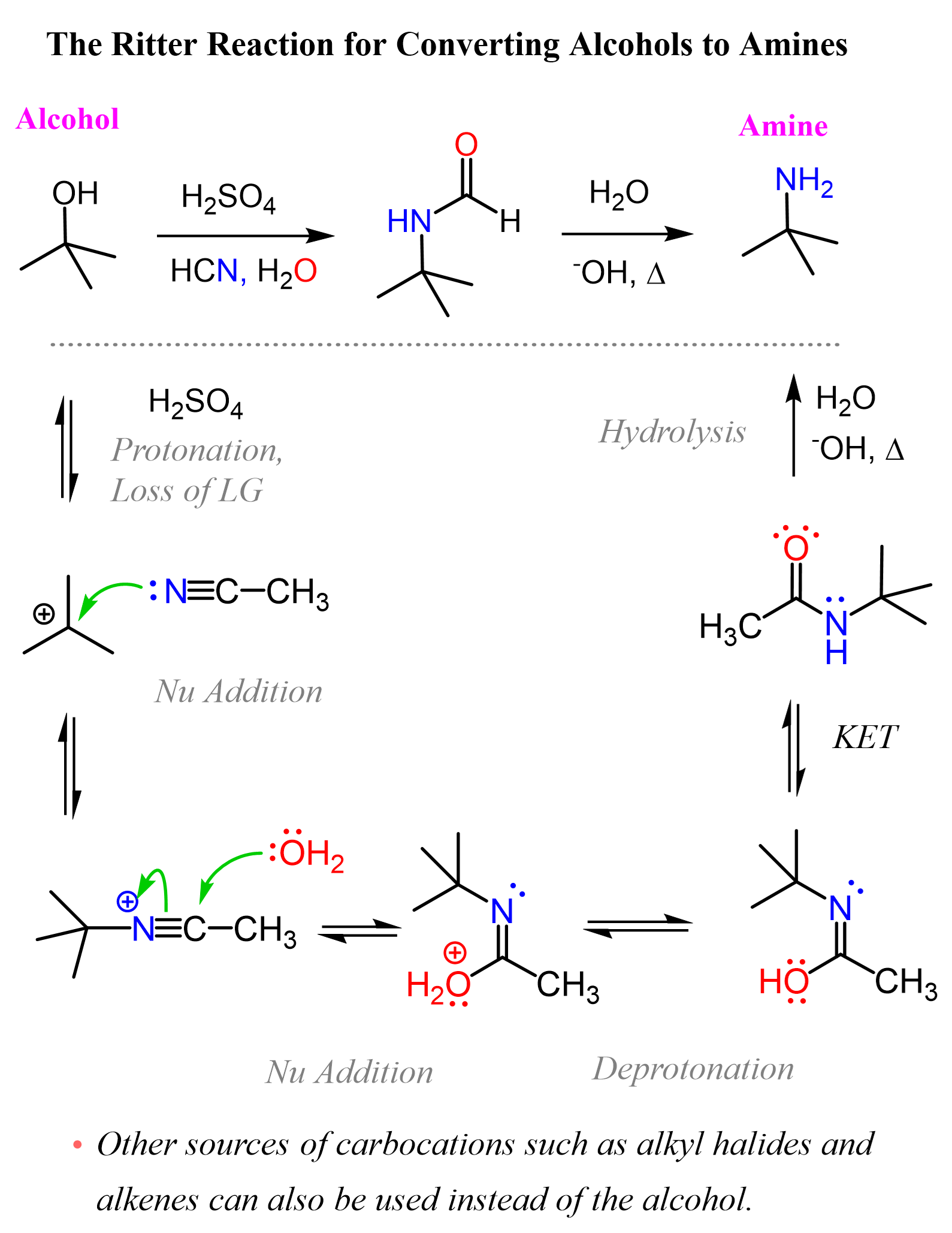
Keep in mind that tertiary and resonance-stabilized alcohols are the best candidates for forming carbocations, but this method will also work with secondary and even primary alcohols when strong Lewis acids are used.
Check Also
- The Gabriel Synthesis of Primary Amines
- The Hofmann Elimination of Amines and Alkyl Fluorides
- Formation and Reactions of Imines and Enamines
- Reductive Amination
- The Reaction of Amines with Nitrous Acid
- Reactions of Amines Practice Problems
- Naming Amines: Systematic and Common Nomenclature
- The Reaction of Amines with Nitrous Acid
- Reactions of Amines Practice Problems
- The Cope elimination
- Basicity of Amines
- Boc Protecting Group for Amines