Reaction with Primary Aliphatic and Aromatic Amines
Primary aliphatic and aromatic amines react with nitrous acid forming arena diazonium salts while the reaction of secondary amines gives N-nitrosamines (R2NN=O).

Now, let’s take a closer look and see how this happens starting with the formation of arena diazonium salts. Nitrous acid does not direclty react with the amino group. It is a very unstable acid and therefore, the reaction is carried out with sodium nitrite (NaNO2) which decompose to form nitrous acid under acidic conditions.
The reactive species in both reactions is the extremely electrophilic nitrosonium ion or nitrosyl cation (+NO), formed in situ from sodium nitrite reacting with a strong acid, such as HCl or H2SO4:
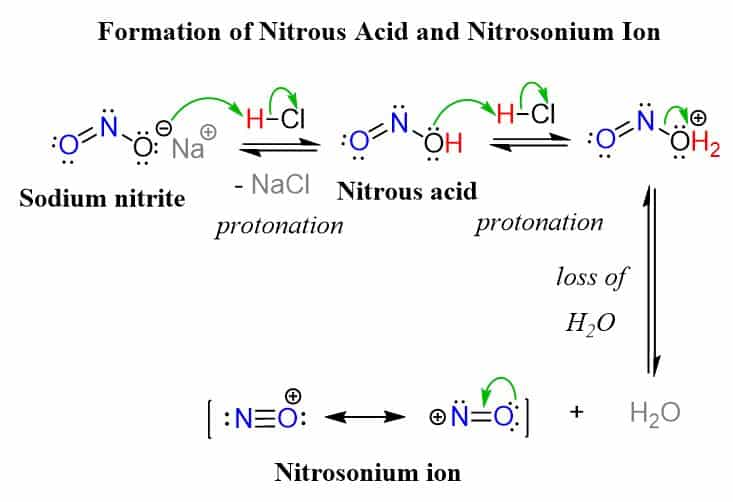
The nitrosonium ion is formed in low concentrations and immediately reacts with the amine present in the reaction mixture thus shifting the equilibrium of this initial step forward. This reaction is called diazotization and begins with nucleophilic attack of the amine on the nitrosonium ion followed by deprotonation. After a proton transfer, an N-nitrosamine is generated which is eventually converted into a diazonium salt by loss of H2O:

Arene diazonium salts represent an important tool in organic chemistry since they’re stable enough to be used as intermediates for preparing substituted benzene rings.
Below is the summary of arene diazonium salt reactions and more details can be found in this post:

Primary alkyl diazonium salts, on the other hand, are not of great interest since they are very unstable below room temperature forming carbocations with loss of N2.
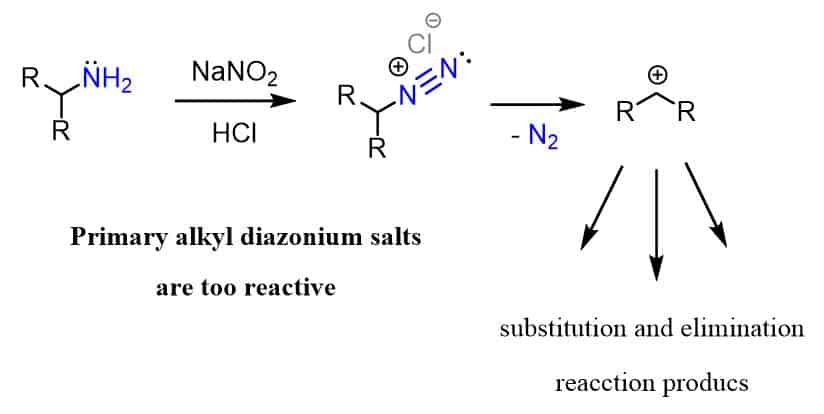
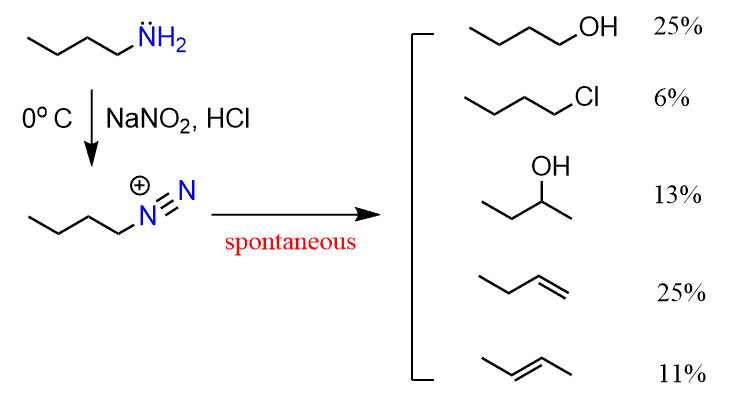
The various substitution and elimination reactions are not directed but rather uncontrollable and therefore, this does not represent a useful synthetic approach. For example, the reaction of butylamine with nitrous acid leads to a mixture of products since the diazonium salt is very unstable:
Now, the question is; how come arene diazonium salts are stable unlike their aliphatic analogs?
The reason for the higher stability of arene diazonium salts is the nature or aryl cations which would have formed if there was loss of nitrogen like in the aliphatic diazonium salts. This would to put the positive charge in an sp2 orbital perpendicular to the conjugated system of p orbitals:
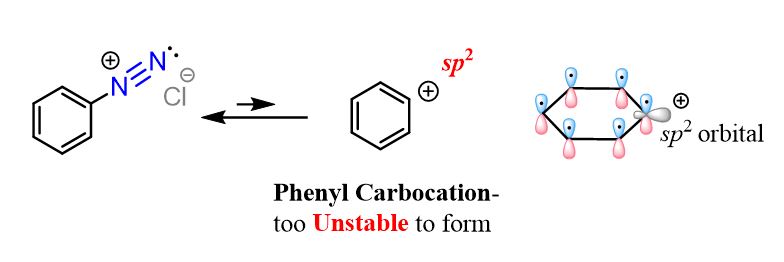
The phenyl cation is very unstable becasue there is no resonance stabilization of the positive charge. Becasue of this, aryl and vinyl carbocations very unfavorable to form and we have seen this, for example, in the Friedel-Crafts reactions which do not work for aryl and vinyl halides for this exact reason.
Reaction of Secondary Amines with Nitrous Acid
The reaction of secondary amines with nitrous acid can be summarized in the first part of the reaction between the amine and nitrosyl cation. The reaction stops at this stage because there is no more proton to be removed and facilitate the loss of water:

This reaction is not nearly as applicable in organic synthesis. However, the product here, a N-Nitrosamines, despite being carcinogens are still used in some foods such as cured meats, bacon, beer, some cheeses to enhance the red color.
Reaction of Tertiary Amines with Nitrous Acid
Tertiary aliphatic amines are only protonated when treated with nitrous acid forming water-soluble salts. This salt does not undergo any reaction and therefore, is of no practical use.
Tertiary aromatic amines can also form salts when treated with nitrous acid. However, in addition to this, they can also undergo electrophilic aromatic substitution since the nitrosyl cation is very electropohilic. This reaction occurs becasueof the strong electron donating ability of the dialkylamino groups. This is a nitrosation and the product is a blue or green aromatic nitroso compounds:
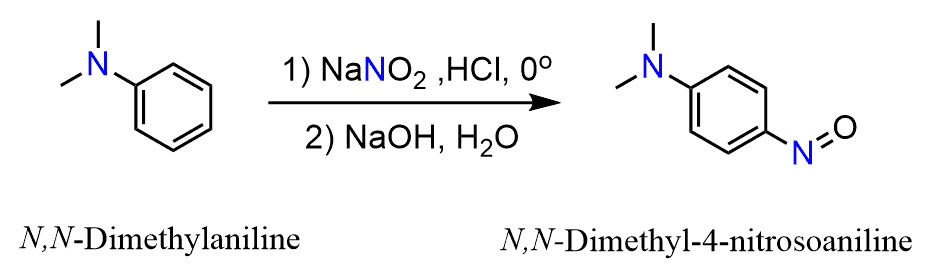
Check Also
- Naming Amines: Systematic and Common Nomenclature
- Preparation of Amines
- The Gabriel Synthesis of Primary Amines
- The Hofmann Elimination of Amines and Alkyl Fluorides
- Imines from Aldehydes and Ketones with Primary Amines
- Enamines from Aldehydes and Ketones with Secondary Amines
- The Reaction of Amines with Nitrous Acid
- The Cope elimination
- Reactions of Amines Practice Problems
- Basicity of Amines

Muito obrigada pela informação
You are welcome:)