Ethers are named by both common and systematic nomenclature of the IUPAC rules. The common names are used for ethers with simple alkyl groups. To do this, we first identify the alkyl groups and arrange them in alphabetical order, followed by the word “ether”.
For example,

The molecules above are examples of unsymmetrical ethers, i.e., different alkyl groups are bridged with the oxygen.
If the groups are identical – symmetrical ethers, the prefix “di” is added. For example,

The Systematic Nomenclature of Ethers
The systematic nomenclature is used for ethers with complex substituents. The idea here is to treat one of the alkoxy (alkyl with the oxygen) groups as a substituent connected to a parent chain. The parent chain is determined just like we always do, based on the longest carbon chain.
For example,

It is important to mention that alkoxy groups do not rank in the priority chart of the functional groups. This means that, just like alkyl groups and halides, they are only treated as substituents and therefore, they do not change the suffix of the parent chain.
For example, let’s compare the effects of the OH and OR groups on naming structurally similar compounds:

The OH group has the highest priority and the parent chain is altered from “ane” to “ol”. The OR group, on the other hand, has no priority and is added as a prefix in alphabetical order.
Now, considering this, let’s name the following ether with alkyl and halide substituents:

Notice again that the ethoxy group has no priority over the methyl and bromide, and they are all added as substituents in alphabetical order.
Check Also
- Preparation of Epoxides
- Ring-Opening Reactions of Epoxides
- Reactions of Epoxides Practice Problems
- The Williamson Ether Synthesis
- Reactions of Ethers-Ether Cleavage
- Nomenclature of Alcohols: Naming Alcohols based on IUPAC Rules with Practice Problems
- Preparation of Alcohols via Substitution or Addition Reactions
- Reaction of Alcohols with HCl, HBr, and HI Acids
- Mesylates and Tosylates as Good Leaving Groups
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in Substitution Reactions Practice Problems
- POCl3 for Dehydration of Alcohols
- Dehydration of Alcohols by E1 and E2 Elimination
- The Oxidation States of Organic Compounds
- LiAlH4 and NaBH4 Carbonyl Reduction Mechanism
- Alcohols from Carbonyl Reductions – Practice Problems
- Grignard Reaction in Preparing Alcohols with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- Protecting Groups For Alcohols in Organic Synthesis
- Oxidation of Alcohols: PCC, PDC, CrO3, DMP, Swern, and All of That
- Diols: Nomenclature, Preparation, and Reactions
- NaIO4 Oxidative Cleavage of Diols
- The Pinacol Rearrangement
- The Williamson Ether Synthesis
- Alcohol Reactions Practice Problems
- Naming Thiols and Sulfides
- Reactions of Thiols
- Alcohols Quiz – Naming, Preparation, and Reactions
- Reactions Map of Alcohols

Shouldn’t the third ether be named propyl-tertbutyl ether as per the alphabetical order?
None of the prefixes such as di, tri, tetra, sec-, tert– are considered for alphabetical priority except the –iso.
You can read this article for more details on IUPAC rules.
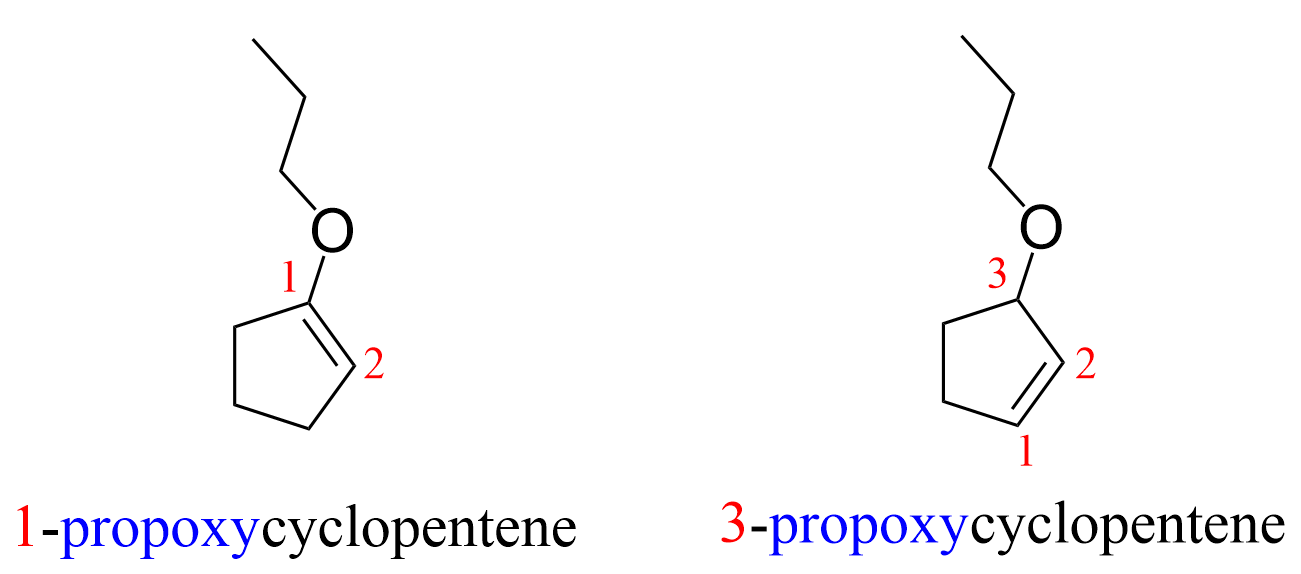
what if you have a cyclopentene with a propoxy group attached
The parent is cyclopentene, which, although does not require a locant to identify the position of the double bond, because it is assumed to be between C1 and C2, you need to make sure the locant for the alkoxy group is assigned correctly. For example, the molecule on the right should not be 2-propoxycyclopentene because in that case the C=C would be between carbons 1 and 5. Other than that, just treat the alkoxy group as an alkyl, or halide – groups that do not take priority over alkenes.
Are positions needed in the common naming of ethers? Or is it just the IUPAC naming?
Not needed in common names – the groups are treated as substituents directly bonded to oxygen.