In the previous post, we talked about the α and β anomers of glucose which are hemiacetals formed as a result of intramolecular cyclization. Anomers are diastereomers and as expected the glucose isomers can be isolated and characterized separately showing different physical properties.
For example, the melting point of α-d-Glucose is 146 °C, while that of β-d-Glucose reaches 150 °C.
An interesting phenomenon is observed when measuring the specific rotation of glucose. When the α anomer is dissolved in water, at first, it exhibits a specific rotation of +112.2 °, but this changes with time to +52.6 °. The same happens with the β anomer and where the specific rotation changes from +18.7 ° to +52.6 °.
So, what is happening here?
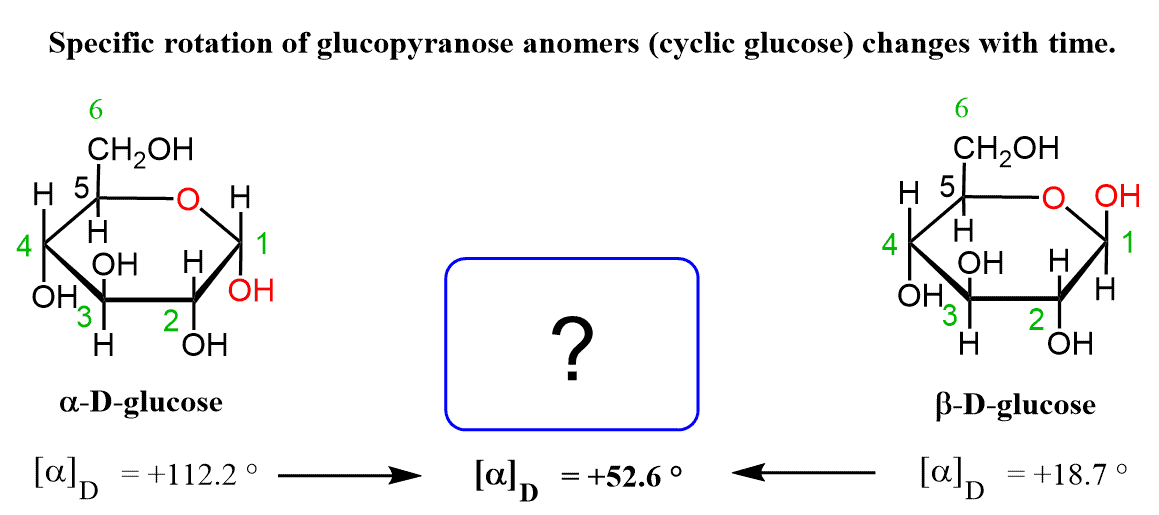
The fact that in both cases the final number is the same +52.6 ° suggests that the molecules undergo a transformation and eventually set at an equilibrium.
Remember, in the introduction article, we mentioned that carbohydrates exist in equilibrium with open and cyclic forms and the latter is favored when 5- or 6-membered rings are possible. So, regardless of what anomer is used, there a little bit of the open-chain glucose that is formed in the solution. The open form of glucose contains an aldehyde and undergoes a cyclization forming both α and β hemiacetals. This phenomenon is called mutarotation and occurs more rapidly when a catalytic amount of acid or base is present.

At equilibrium, the mixture is composed of 37% of the ɑ anomer, 63% of the β anomer, and only trace amounts of the acyclic hydroxy aldehyde. This can be seen by comparing the specific rotation of each anomer to the final value: 52.6o is closer to 18.7o than to 112.2o which indicates the preference for the β isomer in the equilibrium.
As to why the open-chain glucose forms both anomers, this is as a result of a180o rotation about the C1–C2 single bond which places the C=O group pointing up or down. Depending on the orientation of the C5-OH group and the carbonyl, the ɑ-D-glucose or β-D-glucose is formed:

As a final note, let’s also show the mutarotation using the chair forms of glucose since as six-membered rings, the anomers adopt these conformations.
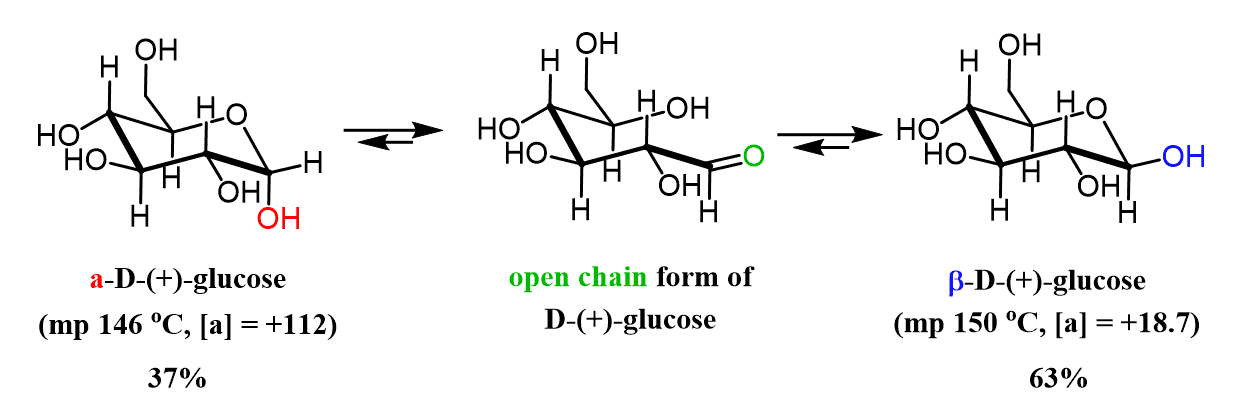
For detailed instruction on converting between Fischer-Haworth-Chair structures of glucose, check the following post: Converting Fischer, Haworth, and Chair forms of Carbohydrates.
Need some practice on carbohydrates?
Check this Multiple-Choice, summary quiz on the structure and reactions of carbohydrates with a 40-min video solution!
Carbohydrates Practice Problem Quiz
Check also in Carbohydrates
- Carbohydrates – Structure and Classification
- Erythro and Threo
- D and L Sugars
- Aldoses and Ketoses: Classification and Stereochemistry
- Epimers and Anomers
- Converting Fischer, Haworth, and Chair forms of Carbohydrates
- Mutarotation
- Glycosides
- Isomerization of Carbohydrates
- Ether and Ester Derivatives of Carbohydrates
- Oxidation of Monosaccharides
- Reduction of Monosaccharides
- Kiliani–Fischer Synthesis
- Wohl Degradation

