Aldoses and ketoses undergo epimerization and other isomerization in basic solutions through keto-enol tautomerization. For example, a solution of D-glucose was found to establish an equilibrium containing both D-glucose and D-mannose:
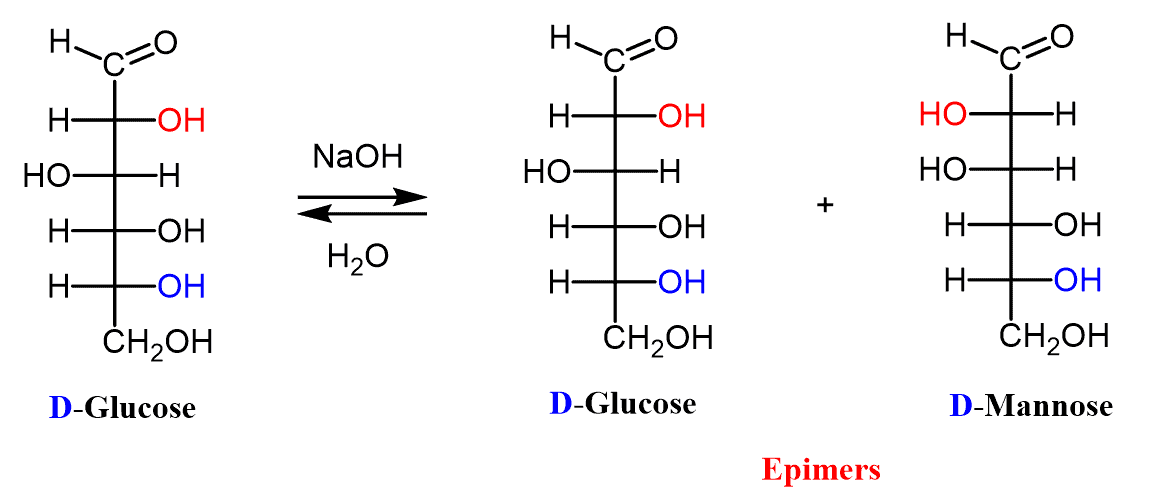
This type of reaction, characteristic of monosaccharides, was first discovered in 1895 by two Dutch chemists, Cornelius Adriaan van Troostenbery Lobry de Bruyn and Willem Alberda van Ekenstein. They are therefore known as Lobry de Bruyn–Alberda van Ekenstein reactions.
Let’s now understand how they happen. First, recall that even though glucose in solution exists mostly in its cyclic hemiacetal forms, traces of the acyclic aldehyde are also present in the equilibrium. Just like any aldehyde, open-chain glucose also has an ɑ carbon and ɑ carbons are associated with acidic hydrogens. Remember, the unusual acidity of ɑ-hydrogen was the basis of enolates formation and reactions. In short, this is due to the electron-withdrawing nature of the carbonyl group which resonance-stabilizes the negative charge of enolates.
Going back to carbohydrates, as much as these structures look huge and expected to exhibit some complicated reactions, it is all about the functional groups present in the molecule.
For example, let’s see the mechanism behind the transformation of glucose to mannose. When exposed to a base, the ɑ carbon of glucose is deprotonated forming an enolate ion:
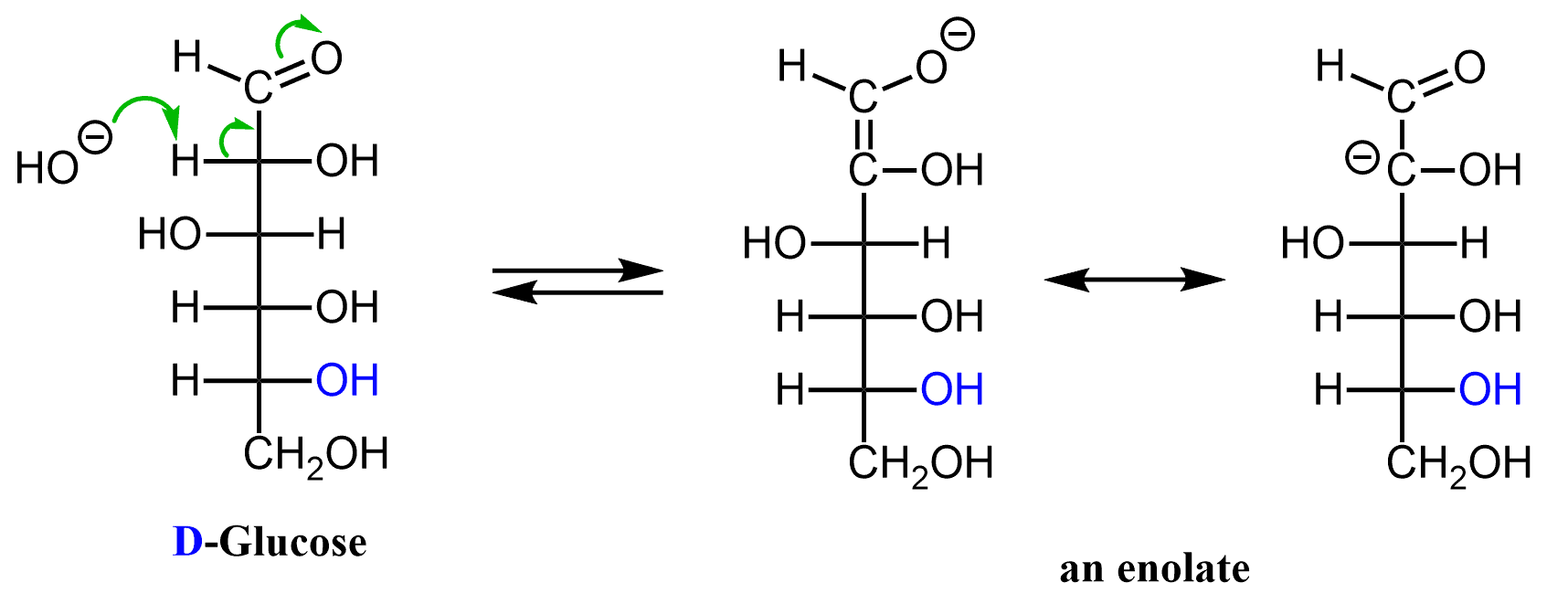
After this, it is simply about the protonation of this enolate. Mannose is formed by protonation at one face of the double bond while protonation at the other face regenerates glucose:

This mixture is also obtained when D-mannose, rather than D-Glucose, is exposed to a base.
If we summarize what happens during this process, we see that one isomer is transformed into two epimers. Remember that D-Glucose and D-mannose are epimers at C-2 because that is the only chiral center with a different configuration. This process is therefore called epimerization and to avoid it when not desired, carbohydrates should not be stored in basic conditions.
Aldose to Ketose Transformation
In the examples above we discussed transformation between different aldoses through the formation of an enolate ion intermediate.
When instead of the carbon, this enolate ion is protonated on oxygen, a new enol, called an enediol (enol with two OH) is formed. This enediol intermediate has two OH groups, and deprotonation of the C-2 OH followed by protonation of C-1 produces fructose:

Deprotonation of the C-1 OH followed by a tautomerization, on the other hand, gives back D-glucose as we have seen earlier.
Need some practice on carbohydrates?
Check this Multiple-Choice, summary quiz on the structure and reactions of carbohydrates with a 40-min video solution!
Carbohydrates Practice Problem Quiz
Check also in Carbohydrates
- Carbohydrates – Structure and Classification
- Erythro and Threo
- D and L Sugars
- Aldoses and Ketoses: Classification and Stereochemistry
- Epimers and Anomers
- Converting Fischer, Haworth, and Chair forms of Carbohydrates
- Mutarotation
- Glycosides
- Isomerization of Carbohydrates
- Ether and Ester Derivatives of Carbohydrates
- Oxidation of Monosaccharides
- Reduction of Monosaccharides
- Kiliani–Fischer Synthesis
- Wohl Degradation

