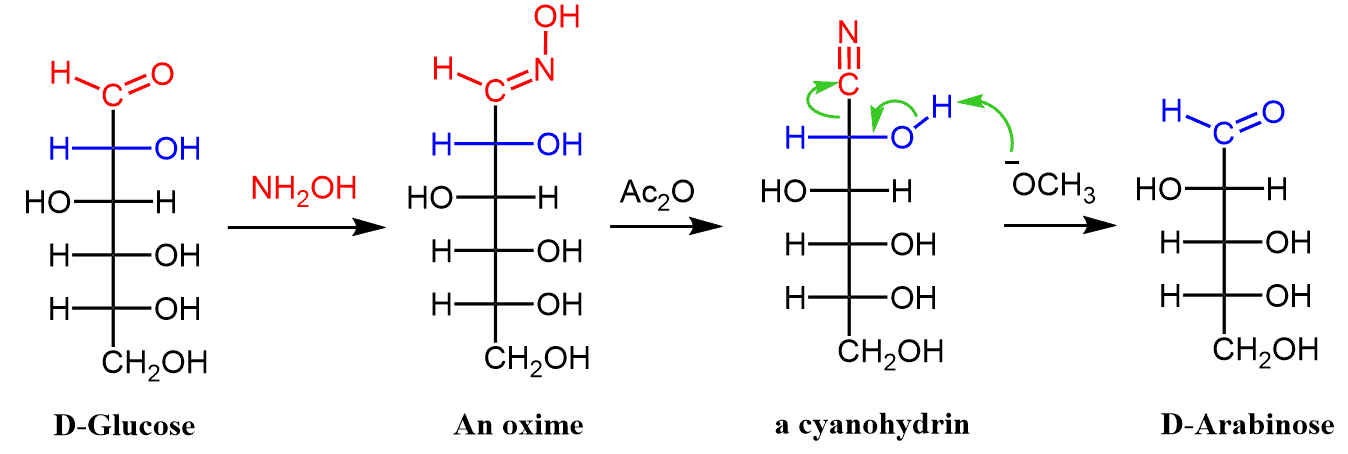
The Wohl degradation cleaves the C1 – C2 bond of an aldose chain and shortens it by one carbon. Like in the Kiliani–Fischer synthesis, the transformation relies on first converting the aldehyde group to a cyanohydrin.

Notice, however, the key difference; the (red) carbon atom of the cyanohydrin group, which is cleaved in the next step, comes from the aldehyde and not the –CN ion like in the Kiliani–Fischer synthesis. Recall that the Kiliani–Fischer synthesis was used to increase the carbon chain by a nucleophilic addition of a cyanide:

So, these two reactions are reverse processes if we compare the net transformations even though the mechanisms are different.
So, let’s discuss the mechanism of Wolf degradation in more detail. The aldehyde is converted into a cyanohydrin via oxime formation as we have seen in the reaction of aldehydes.
Dehydration of the oxime by acetic anhydride produces a cyanohydrin which is kicked out by a strong base forming the new carbohydrate with one less carbon atom:
Need some practice on carbohydrates?
Check this Multiple-Choice, summary quiz on the structure and reactions of carbohydrates with a 40-min video solution!
Carbohydrates Practice Problem Quiz
Check also in Carbohydrates
- Carbohydrates – Structure and Classification
- Erythro and Threo
- D and L Sugars
- Aldoses and Ketoses: Classification and Stereochemistry
- Epimers and Anomers
- Converting Fischer, Haworth, and Chair forms of Carbohydrates
- Mutarotation
- Glycosides
- Isomerization of Carbohydrates
- Ether and Ester Derivatives of Carbohydrates
- Oxidation of Monosaccharides
- Reduction of Monosaccharides
- Kiliani–Fischer Synthesis
- Wohl Degradation


Hi, in the last reaction mechanism, glucose oxime is missing an oxygen atom on nitrogen.
Thanks for spotting that! Fixed.