In the following few posts, we are going to discuss the scary “enthalpy”, “entropy, and “Gibbs free energy” terms tailored toward organic reactions. The links will take to the topics in general chemistry which you can read to refresh these concepts. This article is aimed at making these principles a little easier to understand.
For starters, let’s answer this simple question: What is going to happen if we push the ball just enough to move it from the plateau to the hill?

We know that it is going to roll down the hill and among many reasons that can be brought up to explain why that happens, memorize for the rest of your chemistry course that it happens because the ball goes to a lower energy state.

Any system be that a reaction or a simple physical process naturally tends to go to a lower energy state. Dropping any object, turning the sand watch, avalanche are a few examples of the system going to a lower potential energy state.
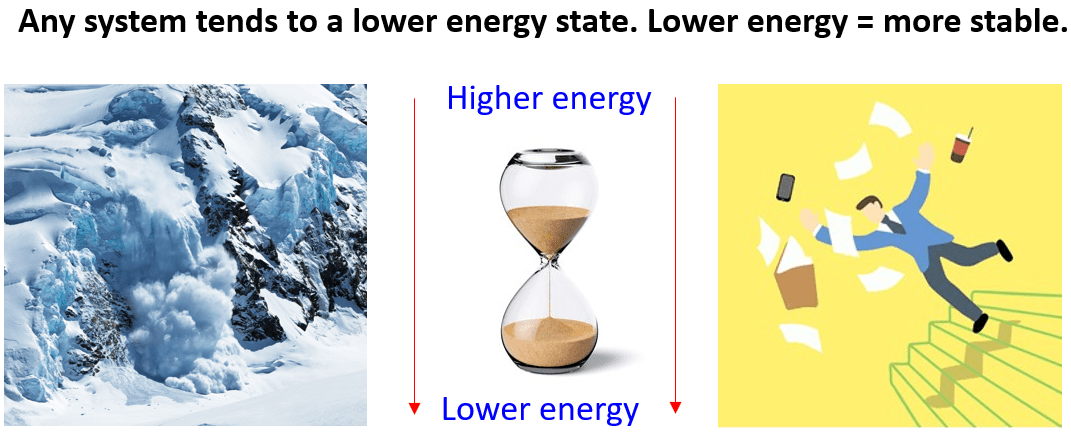
If not interfered with, they tend to move in a direction which is characterized by a lower energy.
Now, what do we mean by interfering here? Interfering means if we want the system to go to a higher energy state, then we need to supply the necessary energy to accomplish that. Going back to the ball, if we want it to go uphill, we must push it up or lift it both of which means providing energy from outside.

The implication of all of this is that lower energy means higher stability and vice versa – the higher the energy the more unstable the system is.
Therefore, molecules or molecular mixtures with lower energy are more stable and less reactive while the ones with higher energy are unstable and more reactive.
Energy and Chemical Reactions
Molecules are not different from other systems in that they have chemical energy which is a form of potential energy that arises from the repulsive electrical forces between the nuclei and electrons. This energy is changed during a chemical reaction and raises or lowered depending on the potential energy of the starting materials and products.
The energy change of a chemical reaction is shown using energy diagrams which are plots of the reaction coordinate (time, progress) and the energy of the starting materials and products.

These curves and notations may not seem as obvious as the images with the ball because the terms enthalpy and free energy are usually marked instead of simply saying energy on the y axis. However, keep in mind that they are all used to convey the same information and that is the energy change during the process/reaction.
For example, the following reaction is an example of a nucleophilic substitution reaction between chloromethane and hydroxide ion:

What we see on the energy diagram is the starting materials have higher protentional energy than the products of the reaction. This means that in the course of the reaction, the molecules have lost some energy. And this energy is lost in a form of heat and heat is the same as the enthalpy, or perhaps it is more accurate to say that enthalpy is the same as heat when the pressure is constant. Now, since most of the reactions are carried out under the same pressure, the heat released or absorbed in a chemical reaction is characterized by the enthalpy change (ΔHo). The Δ (delta) usually means the difference or change and the superscript ° indicates that the measurements are made under standard conditions.
One important thing to note before we move on is that just because the reaction has a tendency to move toward the lower energy state, does not mean that it is going to occur. Just like the ball wasn’t going down the hill unless an initial push was given. This is the activation energy as you may remember from general chemistry.
We will talk about the activation energy and Gibbs free energy explaining spontaneous and nonspontaneous processes in the following articles. However, in this post, let’s focus more on enthalpy to avoid unnecessary confusion.
Negative and Positive Sings of Enthalpy
When the enthalpy is decreasing, it is written with a negative sign and when it is increasing a positive sign is given right before its quantity.
You may sometimes get confused with the negative and positive signs of the enthalpy change and be wondering why is exothermic negative and endothermic positive.
For this, remember that we are always looking from the perspective of the system and in chemistry, the system is the reaction (the molecules). So, if they lose heat then the ΔH must be presented with a negative sign.

If we were to look at the process from the perspective of the surroundings, then we’d say the surrounding has obtained heat and therefore, the enthalpy change would have been positive. So once again, remember that we are always looking from the perspective of the system i.e. the molecules/the reacting species. If they lose/release heat, it is an exothermic reaction (negative ΔHo). If the system absorbs heat, we have an endothermic reaction, and the enthalpy change is characterized by a positive sign.
Why is the Enthalpy Changing During A Reaction?
So far, we have described the change of enthalpy in chemical reactions characterized by negative or positive signs depending on whether the heat is released or absorbed by the system. However, there is an interesting question you may be wondering:
Why is there a change in enthalpy during chemical reactions? Now, to answer this question, we need to go back a little bit in general chemistry and recall that atoms have the highest potential energy when they are free i.e., not bound to each other. Conversely, when there is a chemical bond forming between them, they lose some energy, meaning they go into a more stable state.
For example, the hydrogen molecule H2 is formed when two free atoms of hydrogen come to optimal proximity.
![]()
This process is associated with a 436 kJ mol−1 potential energy loss in the form of heat. The same amount of energy will be needed to break the bond and create two hydrogen atoms (homolytic cleavage).
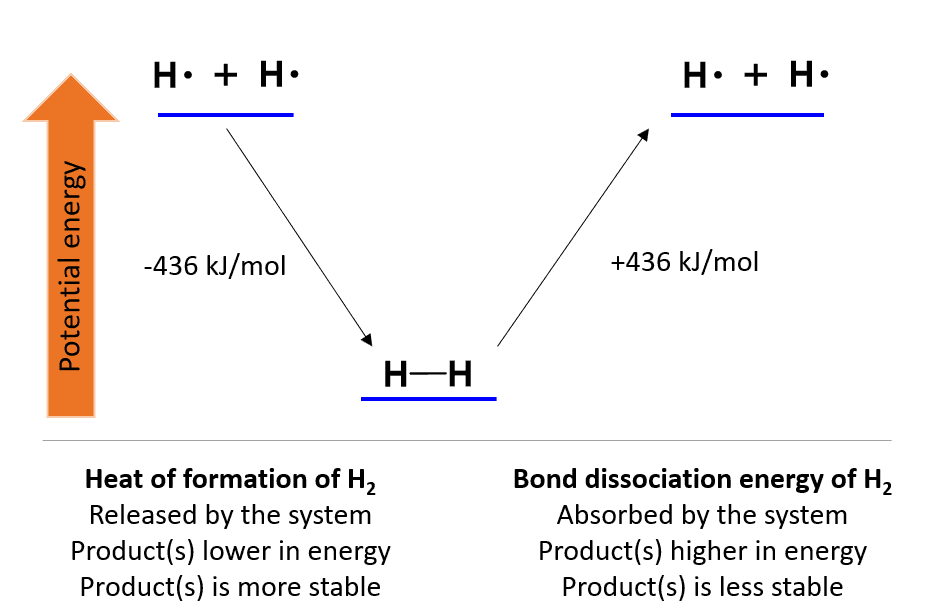
Therefore, this is the H-H bond strength, and the energy needed to break it is called the bond dissociation energy. Contrary, for the reverse process, when H2 is formed, we are talking about the heat of formation and mathematically, these two differ only in their signs.
Just like the H-H bond, the bonds between all the elements are characterized by a specific bond dissociation energy (bond strength).
Now, going back to the question of why there is an enthalpy change during a chemical reaction, we can look at it as the difference between the bond strengths of the starting materials and products. Remember, a chemical reaction is a rearrangement of atoms – some bonds are broken, and new bonds are formed. Each of these bonds requires energy to be broken and each new bond releases energy when atoms are connected. Putting all these values in the summary of the reaction we get the total enthalpy change.
This total change in enthalpy (ΔH°) for the reaction is called the heat of the reaction. And depending on if it is positive or negative, we have endothermic and exothermic reactions respectively.
For example, the following reaction between chlorine and 2-methylpropane is an exothermic reaction ΔH° = −138 kJ/mol.
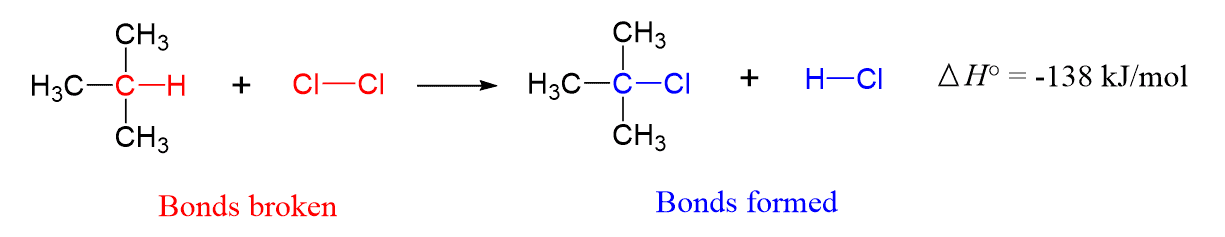
This means more energy is released in forming bonds than is consumed to break the ones in the starting materials. Looking at this from the bond strength perspective, we can say that the bonds formed in the products are stronger than the bonds broken in the starting materials.
Mathematically, the heat of the reaction is calculated by the difference in the sum of ΔH° of bonds broken and the negative sum of the ΔH° of bonds formed.
There is a table with the standard values for the bond dissociation energies and we will discuss using it for determining the heat of the reaction in the next post.
Check Also
