In the previous post, we talked about the main principle of energy, heat and enthalpy associated with chemical reactions. We mentioned, the total change in enthalpy (ΔH°) for the reaction, called the heat of reaction, occurs because of the difference between the bond strengths of the starting materials and products.
Each of the bonds broken during the chemical reaction requires energy while forming a new bond releases energy. And because these energies are not equal, there is a difference when they are summarized for the net transformation.
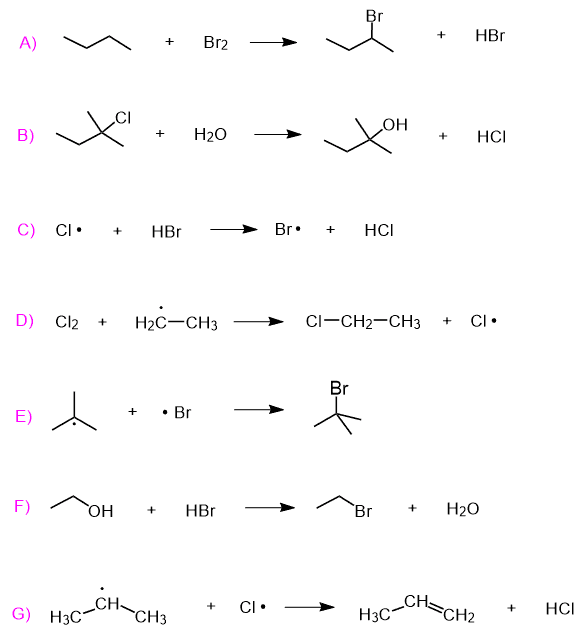
For example, the following reaction between chlorine and 2-methylpropane is an exothermic reaction ΔH° = −138 kJ/mol.
This means more energy is released in forming bonds than is consumed to break the ones in the starting materials. Looking at this from the bond strength’s prospective, we can say that the bonds formed in the products are stronger than the bonds broken in the starting materials.
Let’s look in the table below to see if this conclusion matches with the numeric data of the bond dissociation energies.

The first thing you need to do when working with the bond dissociation energies is to identify the bonds that are broken, and the ones formed during the reaction.
The broken bonds are going to be in the starting materials and the forming bonds are in the products. In this case, they are highlighted in red and blue respectively.
The first broken bond is a C-H bond. However, you need specifically look for the bond connecting the hydrogen to a tertiary carbon.
A quick reminder about primary, secondary and tertiary carbon atoms:

From the tale we find that the ΔH° for (CH3)3C-H bond is 381 kJ/mol. The other bond that is broken is the Cl-Cl which has ΔH° of 243 kJ/mol.
Next, identify the bonds that are formed. The first one is for (CH3)3C-Cl where the ΔH°=331 kJ/mol and the second one is the H-Cl bond (ΔH°=431 kJ/mol).
Now, let’s compare if the bonds formed in the products are stronger than the broken bonds.
ΔH°(bonds formed) = 331 kJ/mol + 431 kJ/mol = 762 kJ/mol
ΔH°(bonds broken) = 381 kJ/mol + 243 kJ/mol = 624 kJ/mol
We can see that ΔH°(bonds formed) > ΔH°(bonds broken). So, the bond dissociation energies of the products are higher (stronger bonds) than the ones of starting materials. Remember, we mentioned that bond formation is a favorable process, and the system loses energy when that happens. And the stronger the bonds, the more energy is released when they are formed. This higher energy released compared to what is absorbed when the bonds are broken, yields to an exothermic reaction.
Sometimes, you don’t need to have this table to determine which bond is going to have a higher bond dissociation energy.
Remember, we talked about the correlation of bond length and bond strength earlier on? What we learned is that the shorter the bond the stronger it is:

As the atoms become larger, the bonds get longer and weaker as well. Longer bonds are a result of larger orbitals which presume a smaller electron density and a poor percent overlap with the s orbital of the hydrogen. Knowing this we can say that the H-F bond is stronger than the H-Cl bond because F is in the second row of the predict table and is smaller than Cl.
Calculating the Heat of Reaction from Bond Dissociation Energies
The heat of the reaction can be calculated with the following formula:

So, in the example above, we’d have to use the following numbers:

A common question when working with enthalpy, energy, heat, and thermodynamics in general is the issue with signs.
For example, why would the sum of the ΔH°(bonds broken) be positive and ΔH°(bonds formed) be negative in the formula for heat of the reaction?
To answer this question, again, you will need to remember that bond formation releasees energy – it is a favorable process. On contrary, breaking bonds requires energy and therefore, external input is needed:
With this said, let’s do one more example.
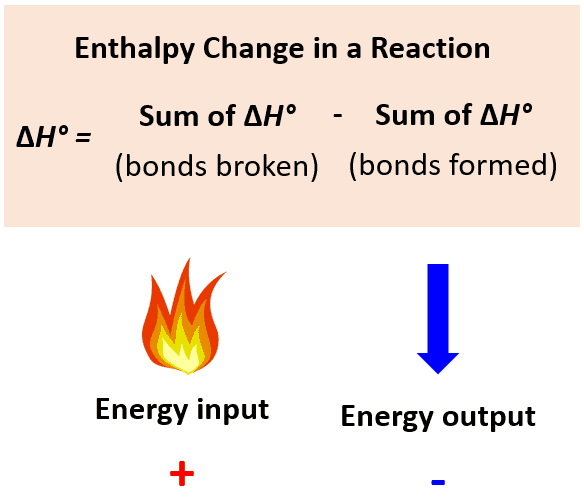
Use the values in the table to calculate the heat of the following reaction and classify it as endothermic or exothermic:
![]()
First, identify the bonds broken and formed:
Next, look up the bond dissociation energies from the table and use the formula to calculate the heat of the reaction:

Do Bond Dissociation Energies Predict the Reactivity of Any Molecule?
Bond dissociation energies are specific to the homolytic cleavage of covalent bonds. However, we can predict the energetic outcome of most organic reactions based on the BDE values regardless if the reaction mechanism is radical or ionic.
It is important to mentioned again that bond dissociation energies do not necessarily reflect the reactivity of functional groups in a given reaction. Other factors such as the acid/base strength, electronegativity of atoms needs to be considered when working with ionic reactions such as nucleophilic substitution and elimination reactions.
For example, the O-H bond dissociation energy is 436 kJ/mol and it can be broken by reacting with a strong base such as the amide ion. In contrary, the C-H bond does not react with sodium amide despite the fact that it is only 381 kJ/mol meaning it is a weaker bond that the O-H bond:

So, how do we explain this observation?
As we mentioned earlier, the bond dissociation energies are measured for the homolytic mechanism where radicals are formed. In the reaction of the alcohol with sodium amide, however, the product is not a radical but an ionic species. More specifically it is the conjugate base of the alcohol and therefore, the reaction is an acid-base reaction. Remember, the acid-base reactions are described by the pKa values of the functional groups.
In this case, the alkoxide ion (conjugate base of the alcohol) is more stable than the carbanion that would have formed if the sodium amide was able to deprotonate (heterolytic cleavage) the alkane.