Preparation of Thiols
Thiols (RSH) are prepared by relying on the high nucleophilicity of sulfur. For example, the reaction of sodium hydrosulfide with unhindered alkyl halides is the most common approach for preparing thiols.
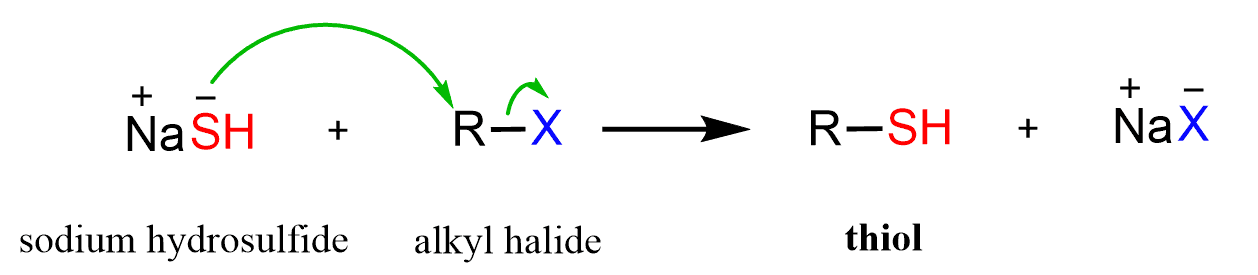
Because of the high nucleophilicity of the thiol, the hydrosulfide is used in a large excess to prevent it from reacting with the alkyl halide and giving a sulfide/thioether (R-S-R).
Another reaction with alkyl halides uses thiourea as a nucleophilic sulfur source, producing alkylisothiouronium salts, which are then hydrolyzed to thiols.

Let’s now talk about the reactions of thiols, starting with their acidity.
Acidity of Thiols
We know that thiols are sulfur analogues of alcohols, containing an SH group in place of the OH group. Therefore, they are going to have some similar features, and meanwhile, there will be reactions that can set them apart.
So, what is the basis of the similarities and differences in the chemistry of thiols and alcohols? Oxygen and sulfur are in the same group of the periodic table, and this gives them some common properties. However, sulfur is in the third row, which makes it a larger atom, and this brings some differences to the chemical properties of thiols and alcohols. Remember that going down a periodic table group, the atomic sizes increase, which helps them handle the negative charge more efficiently because the charge density decreases with large volume/surface.
This is why, despite being less electronegative than oxygen, sulfur stabilizes the negative charge better, and thus thiols are more acidic than alcohols.
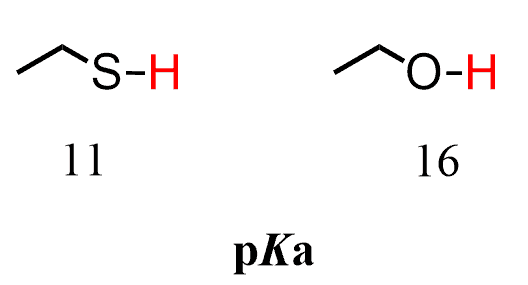
The conjugate base of thiols is the thiolate ion (mercaptides), which has a negative charge on sulfur, and it delocalizes the charge more efficiently than oxygen does in the alkoxide ion.
So, thiols have lower pKa than alcohols, which is to say that thiols are stronger acids than alcohols. In fact, the acidity of the thiol is almost a million times that of the alcohol, and as predicted, thiols are almost entirely deprotonated when treated with a hydroxide base:
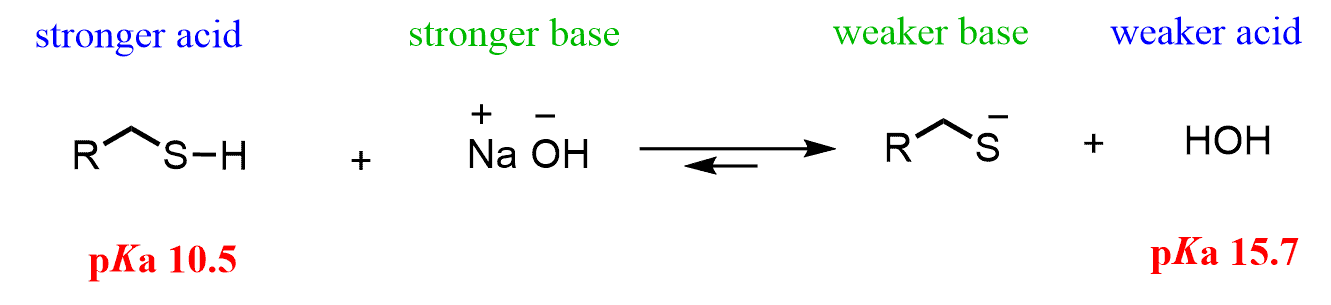
Remember, in acid-base reactions, the equilibrium is shifted to the formation of an acid with a higher pKa since it is a weaker acid. You can review the acid strength and the equilibrium in acid-base reactions if it’s been a while.
We have also discussed the effect of atomic size on stabilizing the negative charge in the post about the stability of resonance structures. If you are comparing elements from different rows in the periodic table, choose the one where the charge is on the bigger atom as the major resonance structure:

Due to its polarizability, the sulfur atom not only readily bears a negative charge but also stabilizes it on an adjacent atom. Therefore, hydrogen atoms on a carbon next to an alkylthio group are more acidic than those the ones adjacent to an oxygen. For example, dimethyl sulfoxide (DMSO) can be deprotonated at the carbon atom when treated with sodium hydride:
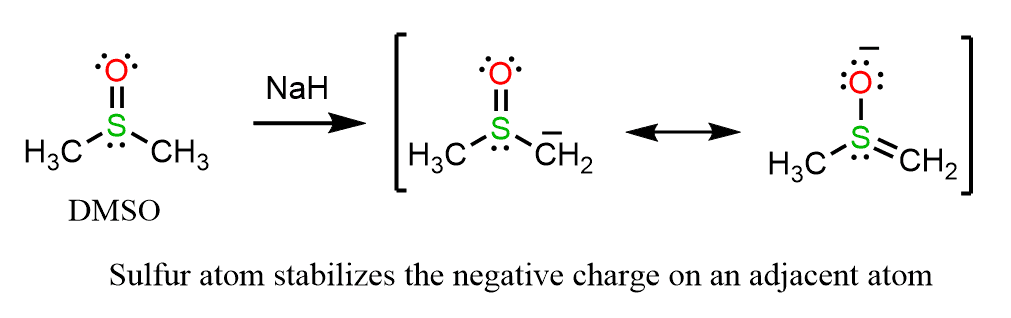
Thiols as Nucleophiles
As mentioned earlier in the preparation reactions, thiols and especially thiolates are great nucleophiles. At the same time, the larger size of the sulfur makes it a weaker base than, for example, oxygen. Remember, alkoxide ions are good nucleophiles and also strong bases, and when reacted with bulkier substrates, the E2 reaction starts dominating.
Alkyl sulfides, on the other hand, perform SN2 on 2o alkyl halides without significantly competing E2 reactions.
Again, this illustrates that sulfur is a good nucleophile but a poor base, while oxygen is a good nucleophile and a strong base.

Of course, the balance of SN2 and E2 competition may depend on individual cases, but going to tertiary alkyl halides is going to favor the E2 pathway even with the thiolate ion.
Check this post to refresh your strategies for choosing between substitution and elimination reactions.
Thiols react with aldehydes and ketones similar to alcohols and form thioacetals, which are less stable compared to acetals.
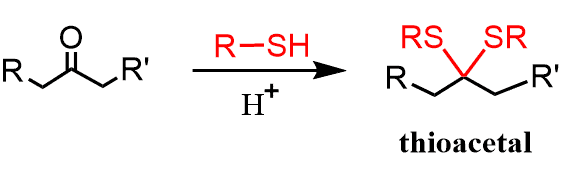
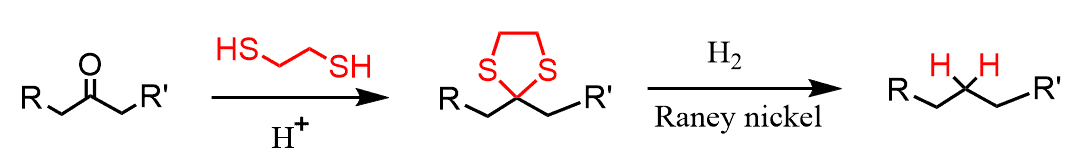
Dithiols, on the other hand, form cyclic thioacetals, which are stable and used in different reactions, including the conversion of the carbonyl to hydrocarbons.
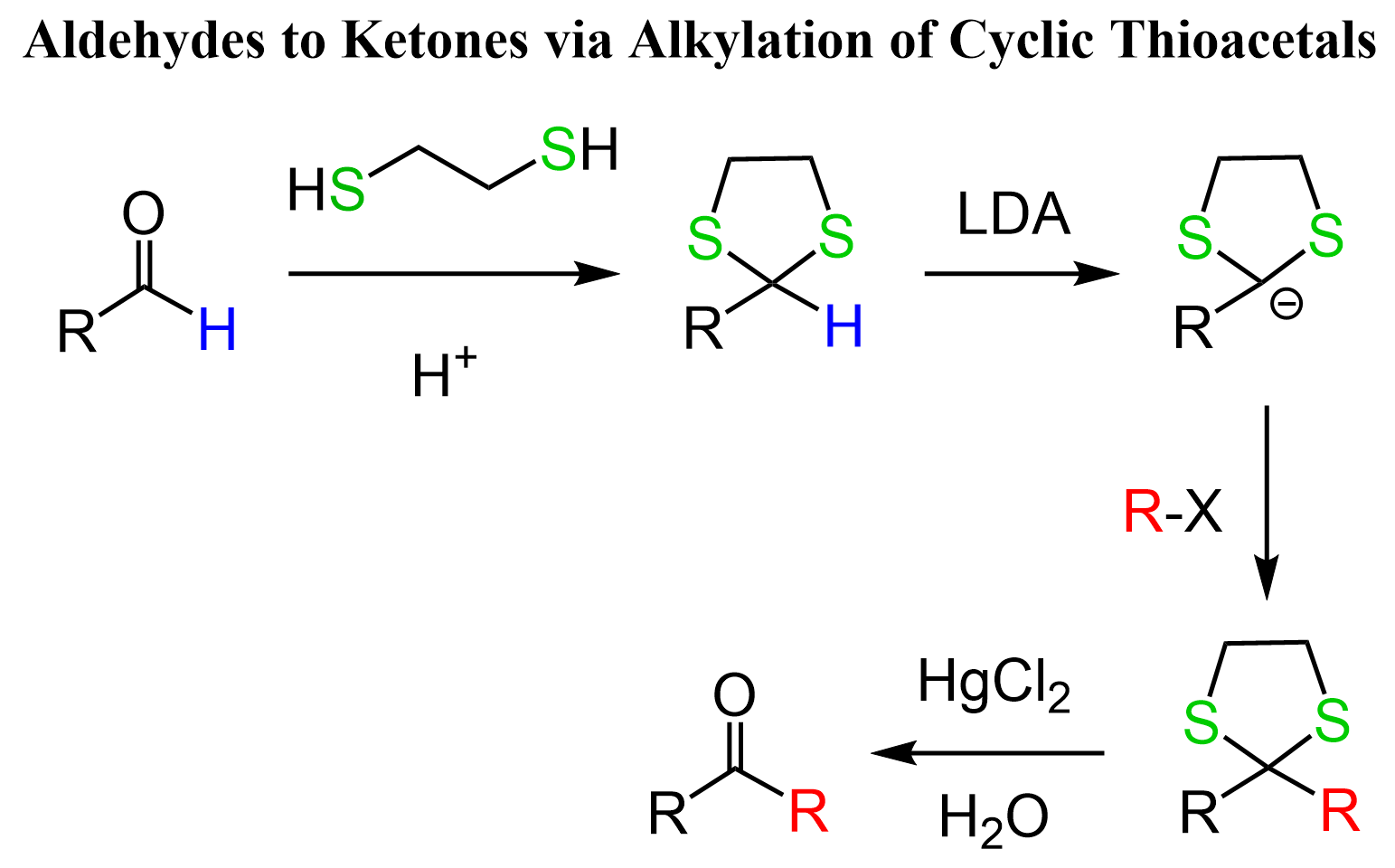
Thioacetals are great species for some synthetic transformations because of the lowered pKa of the carbonyl proton (from ~45 to ~32). They can be deprotonated with strong bases such as LDA or organolithiums, and the resulting carbanion acts as a nucleophile, which allows, for example, converting aldehydes to ketones:
Oxidation of Thiols
Another difference in the reaction of alcohols and thiols comes from the bond strength of thiols. The S-H bond is weaker than the O-H bond, which we can see from the bond dissociation energies (∼365 kJ mol−1) vs (∼430 kJ mol−1). This is explained by the correlation between the bond length and bond strength,h, where we learned that the bond strength gets weaker as it gets longer.
The weakness of the S−H bond brings an important feature to thiols, which is their oxidation to disulfides known as disulfide “bridges”. This process of formation and cleavage of disulfide linkages is an important aspect of protein chemistry, as the disulfide “bridges” between cysteine amino acid residues are responsible for stabilizing the tertiary structure of certain proteins:

The curly hair is a result of disulfide bonds between cysteine amino acid residues, and to make straight hair, these bonds are cleaved by reduction, forming free thiol groups. In the reverse process, the thiols are oxidized to disulfide bridges, thus restoring the curly hair.
Overall, the conversion of thiols to sulfides is an oxidative coupling reaction that can be achieved with mild oxidizing agents such as peroxides, iodine, bromine, and even atmospheric oxygen.
Oxidation of Thiols with Strong Oxidizing Agents
Sulfides can be oxidized further to sulfoxides and then sulfones.
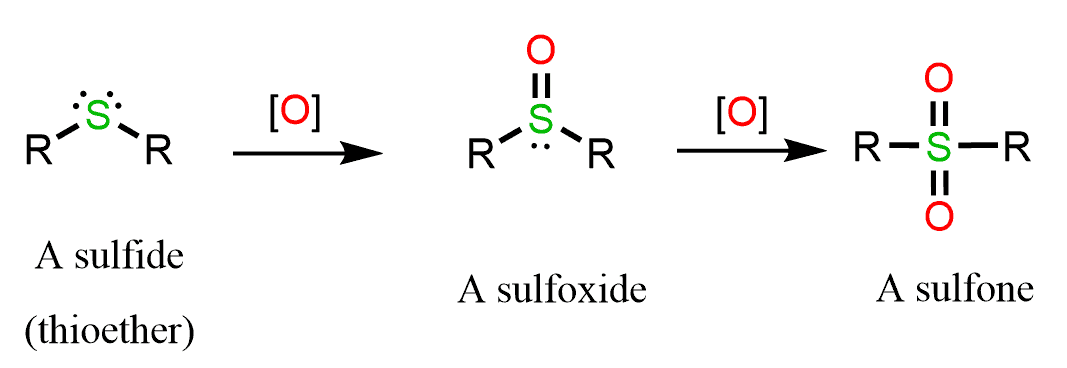
This tendency of sulfides to get easily oxidized makes them ideal reducing agents in different reactions. For example, in ozonolysis, DMS (dimethyl sulfide) is used as a reducing agent.
Sulfur, being a polarizable atom, induces a partial positive charge on one of the connected oxygens and attacks it, cleaving the weak O-O bond. As a result, the carbonyls are formed and the DMS is oxidized to DMSO:

Alcohols, more specifically the oxygen atom, do not undergo such oxidation reactions. It is rather the weaker C-H bond (∼380 kJ mol−1) where the oxidation takes place, and depending on the alcohol and the oxidizing agent, aldehydes, ketones, and carboxylic acids can be formed.
Besides the bond strength, oxygen is in the second row of the periodic table and does not have the necessary d orbitals to exceed the octet, while sulfur does, and with stronger oxidizing agents, sulfinic and sulfonic acids can be formed:
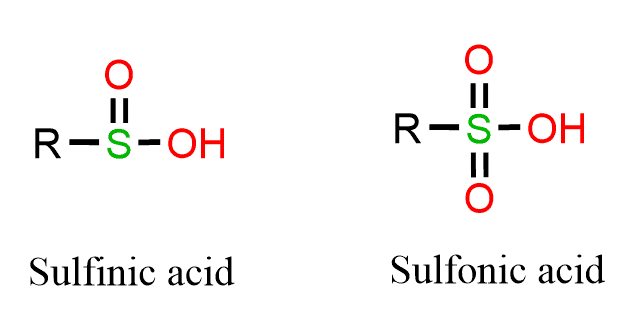
This type of oxidations can be achieved with KMnO4, HNO3 or bleach (NaOCl):
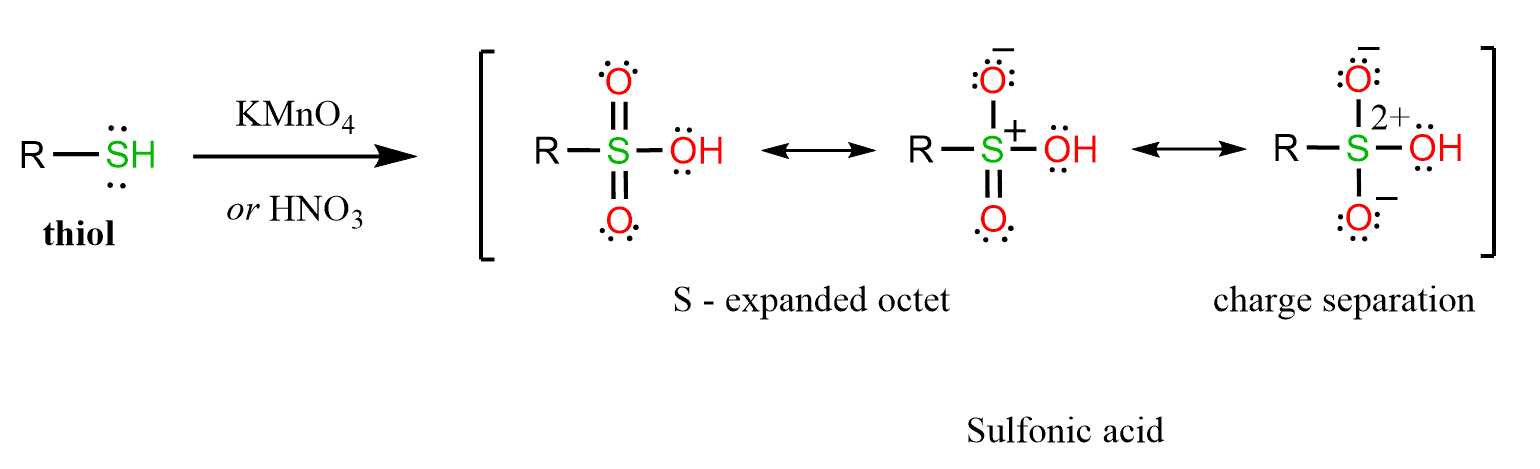
Note that in thiols, the valence shell of sulfur contains 8 electrons, while in sulfinic acid and sulfonic acid, there are 10 and 12 electrons, respectively. This resonance structure can be avoided if the atoms are shown with charge separation, and this is still a subject of debate as to which resonance form is closest to the hybrid structure. This, of course, is not possible with oxygen.
Check also
- Nomenclature of Alcohols: Naming Alcohols based on IUPAC Rules with Practice Problems
- Preparation of Alcohols via Substitution or Addition Reactions
- Reaction of Alcohols with HCl, HBr, and HI Acids
- Mesylates and Tosylates as Good Leaving Groups
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in Substitution Reactions Practice Problems
- POCl3 for Dehydration of Alcohols
- Dehydration of Alcohols by E1 and E2 Elimination
- The Oxidation States of Organic Compounds
- LiAlH4 and NaBH4 Carbonyl Reduction Mechanism
- Alcohols from Carbonyl Reductions – Practice Problems
- Grignard Reaction in Preparing Alcohols with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- Protecting Groups For Alcohols in Organic Synthesis
- Oxidation of Alcohols: PCC, PDC, CrO3, DMP, Swern, and All of That
- Diols: Nomenclature, Preparation, and Reactions
- NaIO4 Oxidative Cleavage of Diols
- The Pinacol Rearrangement
- The Williamson Ether Synthesis
- Alcohol Reactions Practice Problems
- Naming Thiols and Sulfides
- Alcohols Quiz – Naming, Preparation, and Reactions
- Reactions Map of Alcohols
