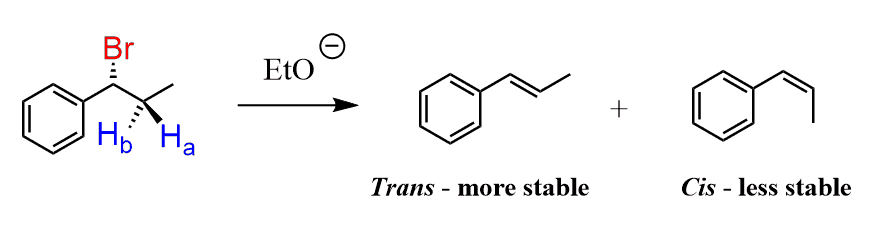
E2 reactions are stereoselective. Let’s understand the meaning of this statement by looking at the following elimination reaction:

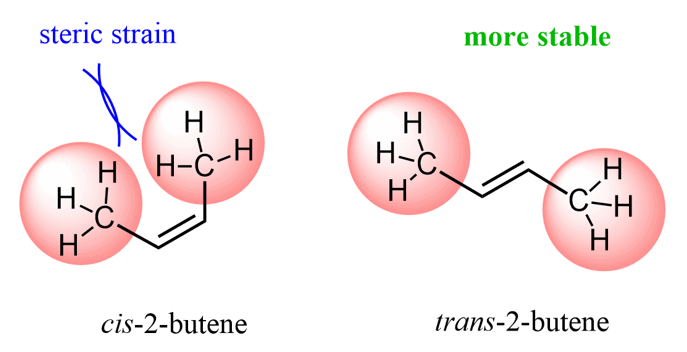
Two stereoisomers are formed – a cis and a trans alkene. The trans alkene is the major product, and this selectivity can be explained by the higher stability of the trans isomer. This essentially is what a stereoselective reaction means – two stereoisomers are possible, but one of them is formed in excess.
However, there is more to this, and to get to the root of this observation, we need to take a closer look at the mechanism of the E2 elimination.
Stereochemistry of the E2 Reaction
The cause of the stereochemistry in E2 reactions is the special alignment of the β-hydrogen and the leaving group in the transition state. The requirement is to have these two groups in one plane – periplanar:

- If the β-hydrogen and the leaving group are on the same side of the molecule, it is called a syn periplanar
- If the β-hydrogen and the leaving group are on opposite sides of the molecule, it is called an anti–periplanar
Most often, the E2 elimination occurs in the anti-periplanar geometry since this is the low-energy staggered conformation of the alkyl halide.
How does the anti-periplanar geometry explain the stereochemistry of the E2 reaction?
Let’s go back to the reaction we talked about above and draw out both β-hydrogens of the alkyl halide as well as assign a configuration to the ɑ-carbon. It does not matter if we make the Br a wedge or a dash.
It is important to mention that there are two β-hydrogens! The number of β-hydrogens is ultimately what explains the stereochemistry of E2 reactions. If there are two of them, then the reaction is stereoselective, whereas the presence of one beta hydrogen leads to a stereospecific reaction since the reactant “does not have a choice”.
To demonstrate the stereoselectivity of the E2 mechanism, we will use the Newman and sawhorse projections of the alkyl halide participating in the E2 elimination. There are two conformations that allow a β-hydrogen to be anti-periplanar to the leaving group:
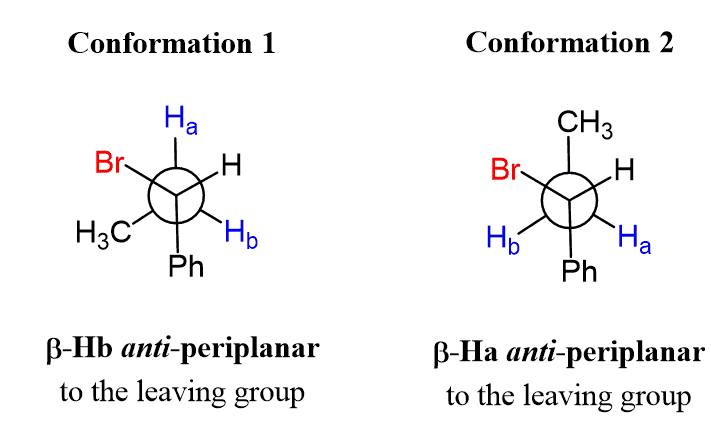
In the first one, it is hydrogen Hb that is anti to the Br, thus participating in the elimination, and in the conformation, it is the Ha that is anti to the Br. We are going to do an E2 elimination based on each conformation and determine the structure of the corresponding alkene.
Conformation 1:
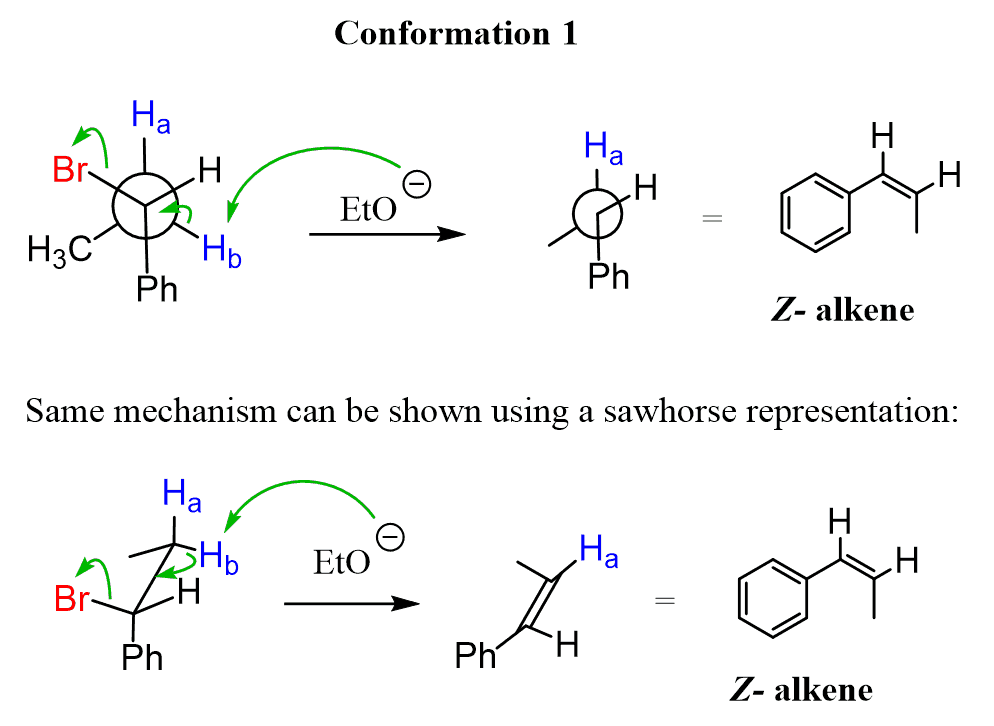
The first conformation leads to the formation of the cis alkene. However, it is not the major product because this conformation is not as stable as the second one. And the reason is the extra gauche interaction between the methyl and phenyl groups as seen in the Newman projection.
Conformation 2:
On the other hand, the second conformation puts the phenyl and methyl groups at anti geometry, and there is only one gauche interaction between the methyl and Br groups. As a result, this more stable conformation leads to the more stable trans alkene as the major product.

To summarize, the E2 elimination is stereoselective because it “selects” to form the more stable stereoisomer.
Most often, you will not be asked to draw the Newman projections to explain the stereoselectivity of an E2 reaction, and to determine the major product, just select the more stable alkene. Recall that trans akenes are generally more stable because of the steric interactions in the cis alkene.
Below is a short summary of how the stereochemistry of E2 reactions is explained:

Notice that this is only true when there are two β-hydrogens and the molecule can choose which one to use in the elimination reaction. I.e., the E2 reaction is stereoselective when there are two β-hydrogens.
The Stereospecificity of E2 reactions
Now, what if there is only one b-hydrogen in the alkyl halide?
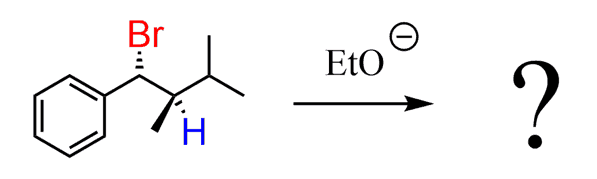
In this case, the reaction is said to be stereospecific since the molecule cannot select a β-hydrogen for the elimination and thus form the more stable alkene. It has no choice, and the structure of the product depends on the structure of the reactant. The stereochemistry depends specifically on the stereochemistry of the reactant.
In this reaction, only the Z alkene is formed, even though it is less stable than the E alkene:
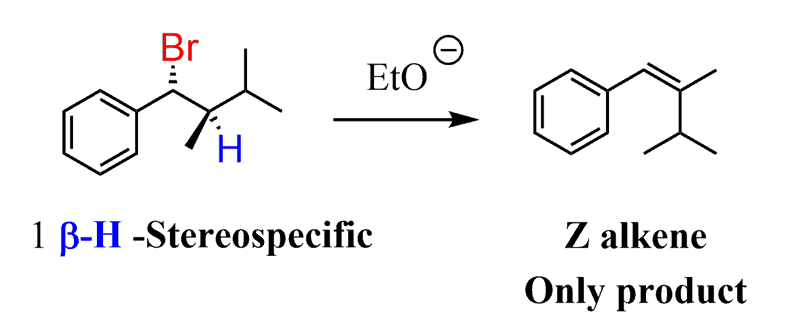
The stereospecificity of the E2 reaction is covered in the next post.