Hydroboration-oxidation converts alkenes into alcohols:

THF (tetrahydrofuran) is the solvent that is used to stabilize the BH3, which otherwise tends to form a dimer, B2H6 – a flammable, toxic, and explosive gas:

It is a few-step transformation that starts from the addition of borane (BH3) to the alkene. This is called hydroboration, and it is an electrophilic addition to the alkene. Boron is a Lewis acid and accepts the electrons of the π bond:

This is a concerted mechanism, meaning that all the bonds are breaking and forming at the same time:

The resulting alkyl borane still has two hydrogen and the reaction repeats two more times, converting it into a trialkyl borane. Although steric factors may prevent this, especially for bulky alkyl groups.

In the second part of the reaction, the trialkyl borane is oxidized into the corresponding alcohol:
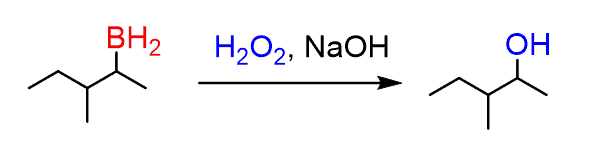
This involves a few steps and starts with the deprotonation of the hydrogen peroxide:
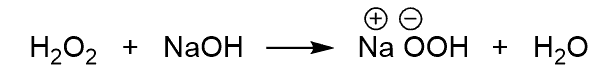
The resulting hydrogen peroxide ion does a nucleophilic attack on the boron:
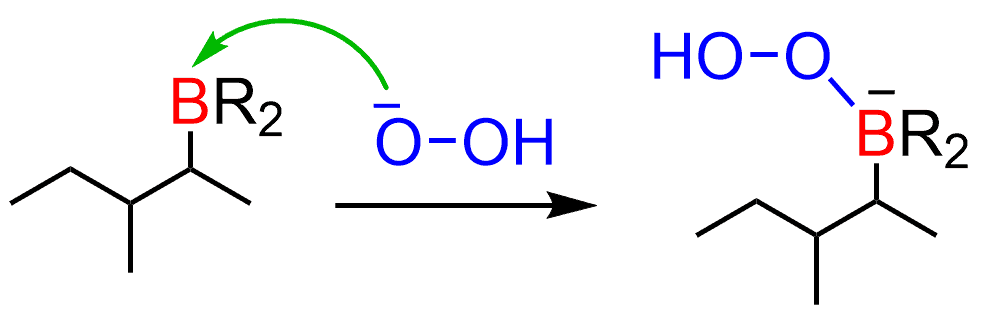
After this, a 1,2-alkyl shift occurs, reducing the electron density on the boron:

Another nucleophilic attack, this time by –OH, followed by loss of the alkoxide ion and its protonation, produces the target alcohol:

In summary, hydroboration-oxidation places an OH group on the less substituted carbon of the alkene:

One to mention here is that for simplicity, the oxidation of alkyl boranes is often shown with the monoalkyl borane instead of the trialkyl borane. However, if BH3 is used, the addition reactions occur first. And because only one H attached to the boron is needed for the entire conversion, commercially available dialkyl boranes are used. The most common one is 9-Borabicyclo[3.3.1]nonane or 9-BBN:

As an example:

📌 Check out this article for a unified mechanism of hydroboration-oxidation of alkenes and alkynes, as well as more examples and practice problems on this method for the hydration of alkenes and alkynes.
Regio and Stereochemistry of Hydroboration-Oxidation
Alkenes can be converted into alcohols by acid-catalyzed hydration, which is more affordable.
The difference between these two reactions is that hydroboration-oxidation allows for anti-Markovnikov addition:

And the advantage is that it is a stereoselective reaction which only does a syn addition of the H and OH to the alkene:

Remember, on the other hand, that acid-catalyzed additions to alkenes are not stereoselective, and the maximum number of stereoisomers is obtained.
Read the next post for the details of Regio and Stereoselectivity of hydroboration-oxidation with practice problems.
Check Also
- Electrophilic Addition Reactions to Alkenes
- Markovnikov’s Rule
- Markovnikov’s Rule with Practice Problems
- Addition of Water to Alkenes
- Acid-Catalyzed Hydration of Alkenes with Practice Problems
- Rearrangements in Alkene Addition Reactions
- Oxymercuration-Demercuration
- Addition of Alcohols to Alkenes
- Free-Radical Addition of HBr: Anti-Markovnikov Addition
- Hydroboration-Oxidation of Alkenes: Regiochemistry and Stereochemistry with Practice Problems
- Halogenation of Alkenes and Halohydrin Formation
- The Regiochemistry of Alkene Addition Reactions
- The Stereochemistry of Alkene Addition Reactions
- Cis product in an anti Addition Reaction of Alkenes
- Ozonolysis of Alkenes with Practice Problems
- Syn Dihydroxylation of Alkenes with KMnO4 and OsO4
- Anti-Dihydroxylation of Alkenes with MCPBA and Other Peroxides with Practice Problems
- Oxidative Cleavage of Alkenes with KMno4 and O3
- Alkene Reactions Practice Problems
- Changing the Position of a Double Bond
- Changing the Position of a Leaving Group
- Alkenes Multi-Step Synthesis Practice Problems
- Alkene Addition Reactions Practice Quiz
- Reactions Map of Alkenes


Isn’t the B negative for the first time the hydrogen peroxide ion does a nucleophilic attack?
Yes, it is. Thanks for pointing that out – fixed.
I have a question about the diborane. So you say THF is used as a solvent to stabilize the diborane but then in figure 2 THF attacks the borane and forms a THF-borane-complex. How does that fit together? Or do you mean stabilize in a sense of breaking it apart into two boranes because the diborane itself is dangerous?
I should have said “stabilize the borane”. Borane is an electron deficient Lewis acid, and therefore, has a tendency to acquire an additional lone pair. In the absence of other Lewis bases, the second molecule of BH3 donates the electrons of the B-H bond forming the B2H6 dimer.
So, it is an equilibrium in which, although the dimer, B2H6 predominates, there is still some BH3 present. Now, because only monomeric borane can undergo a cis addition to olefins, we need to increase its concentration in the solution and for this, an ether solvent such as the THF or any other R2O is used.
When dissolved in these solvents, the role of Lewis base is taken by them as they are stronger electron donors.
We could show a reaction between the dimer and THF forming the THF-BH3 complex, however, the formula of the monomer allows to better illustrate a mechanism for the Lewis acid-base reaction.
Thanks for the comprehensive answer! 🙂
No problem.